Effects of metformin and donepezil on the prevention of doxorubicin-induced cardiotoxicity in breast cancer: a randomized controlled trial
- PMID: 37550350
- PMCID: PMC10406870
- DOI: 10.1038/s41598-023-40061-4
Effects of metformin and donepezil on the prevention of doxorubicin-induced cardiotoxicity in breast cancer: a randomized controlled trial
Abstract
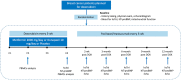
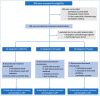
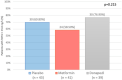
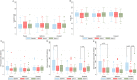
Doxorubicin (DOX) causes deleterious cardiotoxicity. We aimed to investigate the protective roles of metformin and donepezil against DOX-induced cardiotoxicity. In this randomized-controlled trial, 143 female breast cancer patients were enrolled. Metformin (n = 43), donepezil (n = 52), or placebo (n = 48) were prescribed during DOX treatment. The primary endpoint was a proportion of patients with high sensitivity troponin-I (hsTnI) more than the 99th percentile value (> 15.6 ng/L) after DOX treatment. The secondary outcomes were the changes in the hsTnI, N-terminal pro-B-type natriuretic peptide (NT-proBNP), left ventricular ejection fraction (LVEF), global longitudinal strain (GLS) and peripheral blood mononuclear cells analysis for mitochondrial respiration. Baseline characteristics were similar between the groups. The primary endpoint occurred in 58.54% of metformin group, 76.92% in donepezil group, and 69.77% in placebo group (p = 0.215). The level of hsTnI increased after receiving DOX with subsequent decline in LVEF and GLS. Metformin and donepezil did not attenuate hsTnI elevation, LVEF or GLS reduction. There was no significant change in NT-proBNP level. Mitochondrial respiratory dysfunction was observed in the placebo and donepezil groups. However, metformin preserved mitochondrial respiration during DOX therapy. In conclusion, co-treatment with metformin or donepezil did not prevent myocardial injury. Metformin had a favorable mitochondrial outcome and warranted future studies.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Lyon AR, López-Fernández T, Couch LS, et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur. Heart J. 2022;43(41):4229–4361. doi: 10.1093/eurheartj/ehac244. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

