Different niches for stem cells carrying the same oncogenic driver affect pathogenesis and therapy response in myeloproliferative neoplasms
- PMID: 37550517
- PMCID: PMC10447237
- DOI: 10.1038/s43018-023-00607-x
Different niches for stem cells carrying the same oncogenic driver affect pathogenesis and therapy response in myeloproliferative neoplasms
Abstract
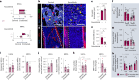
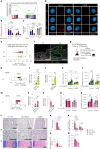
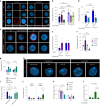
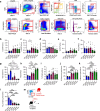
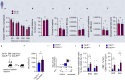
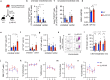
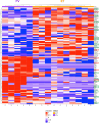
Aging facilitates the expansion of hematopoietic stem cells (HSCs) carrying clonal hematopoiesis-related somatic mutations and the development of myeloid malignancies, such as myeloproliferative neoplasms (MPNs). While cooperating mutations can cause transformation, it is unclear whether distinct bone marrow (BM) HSC-niches can influence the growth and therapy response of HSCs carrying the same oncogenic driver. Here we found different BM niches for HSCs in MPN subtypes. JAK-STAT signaling differentially regulates CDC42-dependent HSC polarity, niche interaction and mutant cell expansion. Asymmetric HSC distribution causes differential BM niche remodeling: sinusoidal dilation in polycythemia vera and endosteal niche expansion in essential thrombocythemia. MPN development accelerates in a prematurely aged BM microenvironment, suggesting that the specialized niche can modulate mutant cell expansion. Finally, dissimilar HSC-niche interactions underpin variable clinical response to JAK inhibitor. Therefore, HSC-niche interactions influence the expansion rate and therapy response of cells carrying the same clonal hematopoiesis oncogenic driver.
© 2023. The Author(s).
Conflict of interest statement
C.N.H. reports funded research from Novartis; speaker fees from Novartis, Janssen, CTI, Celgene and Medscape; and advisory board membership for Incyte, CTI, Sierra Oncology, Novartis, Celgene, Roche, AOP Pharma, Geron and AstraZeneca. A.G. reports speaker fees from Novartis and Bristol Myers Squibb. The other authors declare no competing interests.
Figures
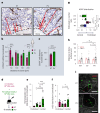
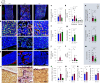
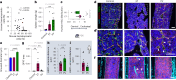
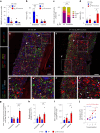







References
-
- Ahmad, H., Jahn, N. & Jaiswal, S. Clonal hematopoiesis and its impact on human health. Annu. Rev. Med.74, 249–260 (2023). - PubMed
-
- James, C. et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature434, 1144–1148 (2005). - PubMed
-
- Kralovics, R. et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N. Engl. J. Med.352, 1779–1790 (2005). - PubMed
-
- Levine, R. L. et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell7, 387–397 (2005). - PubMed
-
- Baxter, E. J. et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet365, 1054–1061 (2005). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

