Lipid nanoparticle-based mRNA delivery systems for cancer immunotherapy
- PMID: 37550567
- PMCID: PMC10406775
- DOI: 10.1186/s40580-023-00385-3
Lipid nanoparticle-based mRNA delivery systems for cancer immunotherapy
Abstract
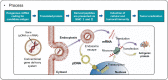
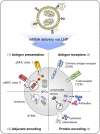
Cancer immunotherapy, which harnesses the power of the immune system, has shown immense promise in the fight against malignancies. Messenger RNA (mRNA) stands as a versatile instrument in this context, with its capacity to encode tumor-associated antigens (TAAs), immune cell receptors, cytokines, and antibodies. Nevertheless, the inherent structural instability of mRNA requires the development of effective delivery systems. Lipid nanoparticles (LNPs) have emerged as significant candidates for mRNA delivery in cancer immunotherapy, providing both protection to the mRNA and enhanced intracellular delivery efficiency. In this review, we offer a comprehensive summary of the recent advancements in LNP-based mRNA delivery systems, with a focus on strategies for optimizing the design and delivery of mRNA-encoded therapeutics in cancer treatment. Furthermore, we delve into the challenges encountered in this field and contemplate future perspectives, aiming to improve the safety and efficacy of LNP-based mRNA cancer immunotherapies.
Keywords: Cancer immunotherapy; Lipid nanoparticles (LNPs); Messenger RNA (mRNA); Tumor-associated antigens (TAAs).
© 2023. Korea Nanotechnology Research Society (KoNTRS).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
