Cross-protein transfer learning substantially improves disease variant prediction
- PMID: 37550700
- PMCID: PMC10408151
- DOI: 10.1186/s13059-023-03024-6
Cross-protein transfer learning substantially improves disease variant prediction
Abstract
Background: Genetic variation in the human genome is a major determinant of individual disease risk, but the vast majority of missense variants have unknown etiological effects. Here, we present a robust learning framework for leveraging saturation mutagenesis experiments to construct accurate computational predictors of proteome-wide missense variant pathogenicity.
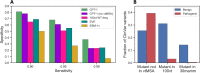
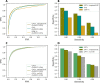
Results: We train cross-protein transfer (CPT) models using deep mutational scanning (DMS) data from only five proteins and achieve state-of-the-art performance on clinical variant interpretation for unseen proteins across the human proteome. We also improve predictive accuracy on DMS data from held-out proteins. High sensitivity is crucial for clinical applications and our model CPT-1 particularly excels in this regime. For instance, at 95% sensitivity of detecting human disease variants annotated in ClinVar, CPT-1 improves specificity to 68%, from 27% for ESM-1v and 55% for EVE. Furthermore, for genes not used to train REVEL, a supervised method widely used by clinicians, we show that CPT-1 compares favorably with REVEL. Our framework combines predictive features derived from general protein sequence models, vertebrate sequence alignments, and AlphaFold structures, and it is adaptable to the future inclusion of other sources of information. We find that vertebrate alignments, albeit rather shallow with only 100 genomes, provide a strong signal for variant pathogenicity prediction that is complementary to recent deep learning-based models trained on massive amounts of protein sequence data. We release predictions for all possible missense variants in 90% of human genes.
Conclusions: Our results demonstrate the utility of mutational scanning data for learning properties of variants that transfer to unseen proteins.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Stenson PD, Ball EV, Mort M, Phillips AD, Shiel JA, Thomas NS, et al. Human gene mutation database (HGMD®): 2003 update. Human Mutation. 2003;21(6):577–581. - PubMed

