GATA2 co-opts TGFβ1/SMAD4 oncogenic signaling and inherited variants at 6q22 to modulate prostate cancer progression
- PMID: 37550764
- PMCID: PMC10408074
- DOI: 10.1186/s13046-023-02745-7
GATA2 co-opts TGFβ1/SMAD4 oncogenic signaling and inherited variants at 6q22 to modulate prostate cancer progression
Abstract
Background: Aberrant somatic genomic alteration including copy number amplification is a hallmark of cancer genomes. We previously profiled genomic landscapes of prostate cancer (PCa), yet the underlying causal genes with prognostic potential has not been defined. It remains unclear how a somatic genomic event cooperates with inherited germline variants contribute to cancer predisposition and progression.
Methods: We applied integrated genomic and clinical data, experimental models and bioinformatic analysis to identify GATA2 as a highly prevalent metastasis-associated genomic amplification in PCa. Biological roles of GATA2 in PCa metastasis was determined in vitro and in vivo. Global chromatin co-occupancy and co-regulation of GATA2 and SMAD4 was investigated by coimmunoprecipitation, ChIP-seq and RNA-seq assays. Tumor cellular assays, qRT-PCR, western blot, ChIP, luciferase assays and CRISPR-Cas9 editing methods were performed to mechanistically understand the cooperation of GATA2 with SMAD4 in promoting TGFβ1 and AR signaling and mediating inherited PCa risk and progression.
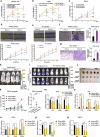
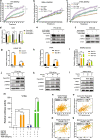
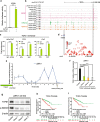
Results: In this study, by integrated genomics and experimental analysis, we identified GATA2 as a prevalent metastasis-associated genomic amplification to transcriptionally augment its own expression in PCa. Functional experiments demonstrated that GATA2 physically interacted and cooperated with SMAD4 for genome-wide chromatin co-occupancy and co-regulation of PCa genes and metastasis pathways like TGFβ signaling. Mechanistically, GATA2 was cooperative with SMAD4 to enhance TGFβ and AR signaling pathways, and activated the expression of TGFβ1 via directly binding to a distal enhancer of TGFβ1. Strinkingly, GATA2 and SMAD4 globally mediated inherited PCa risk and formed a transcriptional complex with HOXB13 at the PCa risk-associated rs339331/6q22 enhancer, leading to increased expression of the PCa susceptibility gene RFX6.
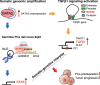
Conclusions: Our study prioritizes causal genomic amplification genes with prognostic values in PCa and reveals the pivotal roles of GATA2 in transcriptionally activating the expression of its own and TGFβ1, thereby co-opting to TGFβ1/SMAD4 signaling and RFX6 at 6q22 to modulate PCa predisposition and progression.
Keywords: GATA2; Prostate cancer; SMAD4; TGFβ1; rs339331.
© 2023. Italian National Cancer Institute ‘Regina Elena’.
Conflict of interest statement
The authors have declared that no competing interests exists.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

