Hypoxic oligodendrocyte precursor cell-derived VEGFA is associated with blood-brain barrier impairment
- PMID: 37550790
- PMCID: PMC10405482
- DOI: 10.1186/s40478-023-01627-5
Hypoxic oligodendrocyte precursor cell-derived VEGFA is associated with blood-brain barrier impairment
Abstract
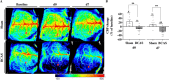
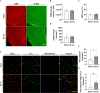
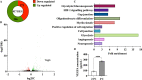
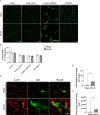
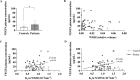
Cerebral small vessel disease is characterised by decreased cerebral blood flow and blood-brain barrier impairments which play a key role in the development of white matter lesions. We hypothesised that cerebral hypoperfusion causes local hypoxia, affecting oligodendrocyte precursor cell-endothelial cell signalling leading to blood-brain barrier dysfunction as an early mechanism for the development of white matter lesions. Bilateral carotid artery stenosis was used as a mouse model for cerebral hypoperfusion. Pimonidazole, a hypoxic cell marker, was injected prior to humane sacrifice at day 7. Myelin content, vascular density, blood-brain barrier leakages, and hypoxic cell density were quantified. Primary mouse oligodendrocyte precursor cells were exposed to hypoxia and RNA sequencing was performed. Vegfa gene expression and protein secretion was examined in an oligodendrocyte precursor cell line exposed to hypoxia. Additionally, human blood plasma VEGFA levels were measured and correlated to blood-brain barrier permeability in normal-appearing white matter and white matter lesions of cerebral small vessel disease patients and controls. Cerebral blood flow was reduced in the stenosis mice, with an increase in hypoxic cell number and blood-brain barrier leakages in the cortical areas but no changes in myelin content or vascular density. Vegfa upregulation was identified in hypoxic oligodendrocyte precursor cells, which was mediated via Hif1α and Epas1. In humans, VEGFA plasma levels were increased in patients versus controls. VEGFA plasma levels were associated with increased blood-brain barrier permeability in normal appearing white matter of patients. Cerebral hypoperfusion mediates hypoxia induced VEGFA expression in oligodendrocyte precursor cells through Hif1α/Epas1 signalling. VEGFA could in turn increase BBB permeability. In humans, increased VEGFA plasma levels in cerebral small vessel disease patients were associated with increased blood-brain barrier permeability in the normal appearing white matter. Our results support a role of VEGFA expression in cerebral hypoperfusion as seen in cerebral small vessel disease.
Keywords: Angiogenesis; BBB; Glial biology; OPC; Vascular dementia; cSVD.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors have no competing interests to declare that are relevant to the content of this article.
Figures

References
-
- Allan KC, Hu LR, Scavuzzo MA, Morton AR, Gevorgyan AS, Cohn EF, Clayton BLL, Bederman IR, Hung S, Bartels CF, Madhavan M, Tesar PJ. Non-canonical targets of HIF1a impair oligodendrocyte progenitor cell function. Cell Stem Cell. 2021;28:257–272.e11. doi: 10.1016/j.stem.2020.09.019. - DOI - PMC - PubMed
-
- Antonetti DA, Barber AJ, Hollinger LA, Wolpert EB, Gardner TW. Vascular endothelial growth factor induces rapid phosphorylation of tight junction proteins occludin and zonula occluden 1. A potential mechanism for vascular permeability in diabetic retinopathy and tumors. J Biol Chem. 1999;274:23463–23467. doi: 10.1074/jbc.274.33.23463. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources

