Collagen peptides supplementation improves function, pain, and physical and mental outcomes in active adults
- PMID: 37551682
- PMCID: PMC10411303
- DOI: 10.1080/15502783.2023.2243252
Collagen peptides supplementation improves function, pain, and physical and mental outcomes in active adults
Abstract
Introduction: Chronic pain affects 19% of adults in the United States, with increasing prevalence in active and aging populations. Pain can limit physical activity and activities of daily living (ADLs), resulting in declined mental and social health. Nutritional interventions for pain currently target inflammation or joint health, but few influence both. Collagen, the most abundant protein in the human body and constituent of the extra cellular matrix, is such a nutraceutical. While there have been reports of reductions in pain with short-term collagen peptide (CP) supplementation, there are no long-term studies specifically in healthy middle-aged active adults.
Purpose: To determine the effects of daily CP consumption over 3, 6, and 9 months on survey measures of pain, function, and physical and mental health using The Knee Injury & Osteoarthritis Outcomes Score (KOOS) and Veterans Rand 12 (VR-12) in middle-aged active adults.
Methods: This study was a double-blind randomized control trial with three treatment groups (Placebo, 10 g/d CP, and 20 g/d CP).
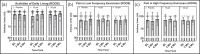
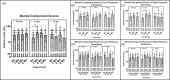
Results: Improvements in ADLs (p = .031, ηp2 = .096) and pain (p = .037, ηp2 = .164) were observed with 10 g/d CP over 6 months, although pain only improved in high frequency exercisers (>180 min/week). Additionally, VR-12 mental component scores (MCS) improved with 10 g/d of CP over 3-9 months (p = .017, ηp2 = .309), while physical component scores (PCS) improved with 20 g/d of CP over 3-9 months, but only in females (p = .013, ηp2= .582).
Conclusion: These findings suggest 10 to 20 g/d of CP supplementation over 6 to 9 months may improve ADLs, pain, MCS, and PCS in middle-aged active adults.
Keywords: Gelatin; KOOS; VR-12; connective tissue; hydrolyzed; nutrition.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
-
- Lopez, HL, Habowski, SM, Sandrock, JE, et al. Effects of dietary supplementation with a standardized aqueous extract of Terminalia chebula fruit (AyuFlex®) on joint mobility, comfort, and functional capacity in healthy overweight subjects: A randomized placebo-controlled clinical trial. BMC Complement Altern Med. 2017;17(1):1–707. doi: 10.1186/s12906-017-1977-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
