Cardiomyocyte external mechanical unloading activates modifications of α-actinin differently from sarcomere-originated unloading
- PMID: 37551968
- PMCID: PMC11285078
- DOI: 10.1111/febs.16925
Cardiomyocyte external mechanical unloading activates modifications of α-actinin differently from sarcomere-originated unloading
Abstract
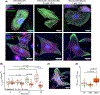
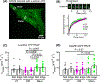
Loss of myocardial mass in a neonatal rat cardiomyocyte culture is studied to determine whether there is a distinguishable cellular response based on the origin of mechano-signals. The approach herein compares the sarcomeric assembly and disassembly processes in heart cells by imposing mechano-signals at the interface with the extracellular matrix (extrinsic) and at the level of the myofilaments (intrinsic). Experiments compared the effects of imposed internal (inside/out) and external (outside/in) loading and unloading on modifications in neonatal rat cardiomyocytes. Unloading of the cellular substrate by myosin inhibition (1 μm mavacamten), or cessation of cyclic strain (1 Hz, 10% strain) after preconditioning, led to significant disassembly of sarcomeric α-actinin by 6 h. In myosin inhibition, this was accompanied by redistribution of intracellular poly-ubiquitin K48 to the cellular periphery relative to the poly-ubiquitin K48 reservoir at the I-band. Moreover, loading and unloading of the cellular substrate led to a three-fold increase in post-translational modifications (PTMs) when compared to the myosin-specific activation or inhibition. Specifically, phosphorylation increased with loading while ubiquitination increased with unloading, which may involve extracellular signal-regulated kinase 1/2 and focal adhesion kinase activation. The identified PTMs, including ubiquitination, acetylation, and phosphorylation, are proposed to modify internal domains in α-actinin to increase its propensity to bind F-actin. These results demonstrate a link between mechanical feedback and sarcomere protein homeostasis via PTMs of α-actinin that exemplify how cardiomyocytes exhibit differential responses to the origin of force. The implications of sarcomere regulation governed by PTMs of α-actinin are discussed with respect to cardiac atrophy and heart failure.
Keywords: HCM; HFrEF; calponin homology domain; myotrope; sarcopenia.
© 2023 The Authors. The FEBS Journal published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
Conflict of interest
RJS is a member of the scientific advisory board of Cytokinetics and a consultant to Pfizer and Edgewise Therapeutics. The remaining authors declare no competing financial interests.
Figures
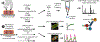




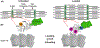
References
-
- Kim GH, Uriel N & Burkhoff D (2018) Reverse remodelling and myocardial recovery in heart failure. Nat Rev Cardiol 15, 83–96. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

