Biosynthetic Plastics as Tunable Elastic and Visible Stent with Shape-Memory to Treat Biliary Stricture
- PMID: 37552006
- PMCID: PMC10582434
- DOI: 10.1002/advs.202303779
Biosynthetic Plastics as Tunable Elastic and Visible Stent with Shape-Memory to Treat Biliary Stricture
Abstract
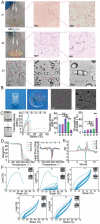
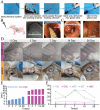
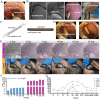
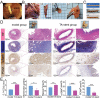
Common biliary tract is ≈4 mm in diameter to deliver bile from liver to small intestine to help digestion. The abnormal narrowing leads to severe symptoms such as pain and nausea. Stents are an effective treatment. Compared with non-degradable stents which require repeated removal, biodegradable stents have the advantage of reducing secondary injury related to endoscopic operation and patient burden. However, current biodegradable materials may cause tissue hyperplasia and the treatment method does not target etiology of stricture. So recurrence rates after biodegradable stent implantation are still high. Here, a biodegradable helical stent fabricated from biosynthetic P(3HB-co-4HB) is reported. Tunable properties can be acquired through altering culture substrates. Stent shows shape memory in various solvents. The stent has an optimized design with helical structure and outer track. The self-expanding of helical structure and double drainage realized by outer track greatly improve drainage of bile. Importantly, stent-loading triamcinolone acetonide can inhibit proliferation of fibroblasts and reduce incidence of restricture. Therapeutic effect is also demonstrated in minipigs with biliary stricture. The results of minipig experiments show that biliary duct in treatment group is unobstructed and tissue hyperplasia is effectively inhibited.
Keywords: biliary stricture; biodegradable stent; endoscopy; polyhydroxyalkanoate; shape memory; triamcinolone acetonide.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- a) Almeida G. G., Donato P., Eur. J. Radiol. 2020, 125, 108899; - PubMed
- b) Hu B., Sun B., Cai Q., Lau J. Y. W., Ma S., Itoi T., Moon J. H., Yasuda I., Zhang X., Wang H.‐P., Ryozawa S., Rerknimitr R., Li W., Kutsumi H., Lakhtakia S., Shiomi H., Ji M., Li X., Qian D., Yang Z., Zheng X., Gastrointest. Endosc. 2017; - PubMed
- c) Vitale G. C., Tran T. C., Davis B. R., Vitale M., Vitale D., Larson G., J. Am. Coll. Surg. 2008, 206, 918. - PubMed
-
- Ramchandani M., Lakhtakia S., Costamagna G., Tringali A., Püspöek A., Tribl B., Dolak W., Devière J., Arvanitakis M., van der Merwe S., Laleman W., Ponchon T., Lepilliez V., Gabbrielli A., Bernardoni L., Bruno M. J., Poley J. W., Arnelo U., Lau J., Roy A., Bourke M., Kaffes A., Neuhaus H., Peetermans J., Rousseau M., Reddy D. N., Gastroenterology 2021, 161, 185. - PubMed
-
- Kaffes A., Endoscopy 2019, 51, 1115. - PubMed
-
- Lawrence C., Romagnuolo J., Payne K. M., Hawes R. H., Cotton P. B., Gastrointest. Endosc. 2010, 72, 558. - PubMed
-
- Dumonceau J. M., Tringali A., Papanikolaou I. S., Blero D., Mangiavillano B., Schmidt A., Vanbiervliet G., Costamagna G., Devière J., García‐Cano J., Gyökeres T., Hassan C., Prat F., Siersema P. D., van Hooft J. E., Endoscopy 2018, 50, 910. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
