Leaf-level metabolic changes in response to drought affect daytime CO2 emission and isoprenoid synthesis pathways
- PMID: 37552065
- PMCID: PMC10643046
- DOI: 10.1093/treephys/tpad094
Leaf-level metabolic changes in response to drought affect daytime CO2 emission and isoprenoid synthesis pathways
Abstract
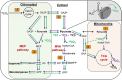
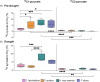
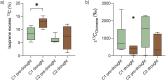
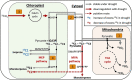
In the near future, climate change will cause enhanced frequency and/or severity of droughts in terrestrial ecosystems, including tropical forests. Drought responses by tropical trees may affect their carbon use, including production of volatile organic compounds (VOCs), with implications for carbon cycling and atmospheric chemistry that are challenging to predict. It remains unclear how metabolic adjustments by mature tropical trees in response to drought will affect their carbon fluxes associated with daytime CO2 production and VOC emission. To address this gap, we used position-specific 13C-pyruvate labeling to investigate leaf CO2 and VOC fluxes from four tropical species before and during a controlled drought in the enclosed rainforest of Biosphere 2 (B2). Overall, plants that were more drought-sensitive had greater reductions in daytime CO2 production. Although daytime CO2 production was always dominated by non-mitochondrial processes, the relative contribution of CO2 from the tricarboxylic acid cycle tended to increase under drought. A notable exception was the legume tree Clitoria fairchildiana R.A. Howard, which had less anabolic CO2 production than the other species even under pre-drought conditions, perhaps due to more efficient refixation of CO2 and anaplerotic use for amino acid synthesis. The C. fairchildiana was also the only species to allocate detectable amounts of 13C label to VOCs and was a major source of VOCs in B2. In C. fairchildiana leaves, our data indicate that intermediates from the mevalonic acid (MVA) pathway are used to produce the volatile monoterpene trans-β-ocimene, but not isoprene. This apparent crosstalk between the MVA and methylerythritol phosphate pathways for monoterpene synthesis declined with drought. Finally, although trans-β-ocimene emissions increased under drought, it was increasingly sourced from stored intermediates and not de novo synthesis. Unique metabolic responses of legumes may play a disproportionate role in the overall changes in daytime CO2 and VOC fluxes in tropical forests experiencing drought.
Keywords: GC–IRMS; PTR–TOF–MS; daytime respiration; legumes; position-specific isotope labeling; tropical plants; volatile organic compounds.
© The Author(s) 2023. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Conflict of interest statement
None declared.
Figures




References
-
- Atkinson R, Arey J (2003) Gas-phase tropospheric chemistry of biogenic volatile organic compounds: a review. Atmos Environ 37:197–219.
-
- Bartoli CG, Gomez F, Martinez DE, Guiamet JJ (2004) Mitochondria are the main target for oxidative damage in leaves of wheat (Triticum aestivum L.). J Exp Bot 55:1663–1669. - PubMed
-
- Bick JA, Lange BM (2003) Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: unidirectional transport of intermediates across the chloroplast envelope membrane. Arch Biochem Biophys 415:146–154. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

