Pacritinib is a potent ACVR1 inhibitor with significant anemia benefit in patients with myelofibrosis
- PMID: 37552106
- PMCID: PMC10561048
- DOI: 10.1182/bloodadvances.2023010151
Pacritinib is a potent ACVR1 inhibitor with significant anemia benefit in patients with myelofibrosis
Abstract
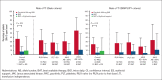
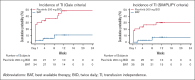
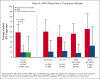
In patients with cytopenic myelofibrosis, treatment with the JAK2/IRAK1 inhibitor pacritinib was associated with anemia benefit in the phase 3 PERSIST-2 study. The impact of pacritinib on transfusion independence (TI) has not been previously described, nor has the mechanism by which pacritinib improves anemia been elucidated. Because it has been previously postulated that inhibition of activin A receptor, type 1 (ACVR1)/activin receptor-like kinase-2 improves anemia in patients with myelofibrosis via suppression of hepcidin production, we assessed the relative inhibitory potency of pacritinib compared with other JAK2 inhibitors against ACVR1. Pacritinib inhibited ACVR1 with greater potency (half-maximal inhibitory concentration [IC50] = 16.7 nM; Cmax:IC50 = 12.7) than momelotinib (IC50 = 52.5 nM; Cmax:IC50 = 3.2), fedratinib (IC50 = 273 nM; Cmax:IC50 = 1.0), or ruxolitinib (IC50 > 1000; Cmax:IC50 < 0.01). Pacritinib's inhibitory activity against ACVR1 was corroborated via inhibition of downstream SMAD signaling in conjunction with marked suppression of hepcidin production. Among patients on PERSIST-2 who were not transfusion independent at baseline based on Gale criteria, a significantly greater proportion achieved TI on pacritinib compared with those treated on best available therapy (37% vs 7%, P = .001), and significantly more had a ≥50% reduction in transfusion burden (49% vs 9%, P < .0001). These data indicate that the anemia benefit of the JAK2/IRAK1 inhibitor pacritinib may be a function of potent ACVR1 inhibition.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: S.T.O. has consulted for AbbVie, Blueprint Medicines, Celgene/Bristol Myers Squibb (BMS), Constellation Pharmaceuticals, CTI BioPharma Corp., Disc Medicine, Geron, Incyte, Cogent, and Sierra Oncology; and has received research funding from Actuate Therapeutics, Blueprint Medicines, Celgene/BMS, Constellation Pharmaceuticals, CTI BioPharma Corp, Incyte, Kartos Therapeutics, Sierra Oncology, and Takeda. R.A.M. has acted in a consulting or advisory role for Constellation Pharmaceuticals, La Jolla Pharmaceutical, Novartis, and Sierra Oncology; has received research support from AbbVie, Celgene, Constellation Pharmaceuticals, CTI BioPharma Corp, Genotech, Incyte, Promedior, and Samus; and has received grants from Mays Cancer Center P30 Cancer Center Support Grant from the National Cancer Institute (CA054174). C.N.H. received honoraria from AbbVie, CTI BioPharma Corp, Geron, Janssen, and Novartis; has served in consulting/advisory capacity for AOP, Celgene/BMS, Constellation Pharmaceuticals, CTI BioPharma Corp, Galecto, Geron, Gilead, Janssen, Keros, Promedior, Roche, Shire, Sierra Oncology, and Novartis; has served on a speakers’ bureau for AbbVie, BMS, CTI BioPharma Corp, Geron, Sierra Oncology, and Novartis; and has received research funding from BMS, Constellation Pharmaceuticals, and Novartis. P.B. has received research support from CTI BioPharma Corp, Disc Medicine, Incyte Corporation, BMS, Blueprint Medicines Corporation, Cogent Biosciences, Morphosys, Kartos Therapeutics, Ionis Pharmaceuticals, Pfizer, Sumitomo Pharma, Telios, Astellas, NS Pharma and Promedior; and honoraria/consulting fees from CTI BioPharma Corp, Incyte Corporation, BMS, Blueprint Medicines Corporation, Cogent Biosciences, Morphosys, Kartos Therapeutics, AbbVie, GSK, Karyopharm Therapeutics, Novartis, and PharmaEssentia. A.T.G. has acted in a consulting or advisory role for AbbVie, BMS, Constellation Pharmaceuticals, CTI BioPharma Corp, Novartis, PharmaEssentia, and Sierra Oncology. V.G. has consulted for AbbVie, Celgene/BMS, Constellation Pharmaceuticals, Novartis, Pfizer, and Sierra Oncology; he has received honoraria from Celgene/BMS, Constellation Pharmaceuticals, and Novartis; and has served in consulting/advisory capacity for AbbVie, Celgene/BMS, Pfizer, and Roche. B.L.S. has consulted for Acceleron Pharma, Celgene, and Novartis; has served on speakers’ bureaus for Alexion Pharmaceuticals, Celgene, Jazz Pharmaceuticals, and Novartis; has received honoraria from BMS, Incyte, and Taiho Oncology; and reports his institution receiving research funding from Celgene. J.-J.K. has acted in a consulting or advisory role for AbbVie, BMS, Incyte, Novartis, and PharmaEssentia. A.L. has consulted for and participated on speakers’ bureaus for Amgen, Grifols, Novartis, and Sanofi; has participated on speakers’ bureaus for BMS Incyte, Pfizer, and SOBI; and has consulted for Morphosys. S.A.B., S.T., B.G.H., K.R.-T., J.S., and A.R.C. are employed at, and own stock in, CTI BioPharma Corp. J.M. has acted in a consulting or advisory role for AbbVie, BMS, Celgene, Constellation Pharmaceutical, CTI BioPharma Corp, Galecto, Geron, Incyte, Kartos, Karyopharm, Novartis, PharmaEssentia, Prelude Therapeutics, and Sierra Oncology; and has received funding to their institution from AbbVie, BMS, Celgene, CTI BioPharma Corp, Forbius, Geron, Incyte, Imago, Kartos, Merck, Novartis, PharmaEssentia, and Roche. S.V. has consulted for BMS, Constellation Pharmaceuticals, Incyte, Novartis, and Sierra Oncology; and has received researching funding from AstraZeneca, Blueprint Medicines, Celgene, CTI BioPharma Corp, Genentech, Gilead, Incyte, Italfarmaco, Novartis, NS Pharma Inc, PharmaEssentia, Promedior, Protagonist Therapeutic, Roche, and Sierra Oncology. T.K. declares no competing financial interests.
Figures




References
-
- Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–2901. - PubMed
-
- Pardanani A, Harrison C, Cortes JE, et al. Safety and efficacy of fedratinib in patients with primary or secondary myelofibrosis: a randomized clinical trial. JAMA Oncol. 2015;1(5):643–651. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

