Overview of the current use of levosimendan in France: a prospective observational cohort study
- PMID: 37552372
- PMCID: PMC10409690
- DOI: 10.1186/s13613-023-01164-3
Overview of the current use of levosimendan in France: a prospective observational cohort study
Erratum in
-
Correction: Overview of the current use of levosimendan in France: a prospective observational cohort study.Ann Intensive Care. 2023 Sep 29;13(1):94. doi: 10.1186/s13613-023-01193-y. Ann Intensive Care. 2023. PMID: 37770809 Free PMC article. No abstract available.
Abstract
Background: Following the results of randomized controlled trials on levosimendan, French health authorities requested an update of the current use and side-effects of this medication on a national scale.
Method: The France-LEVO registry was a prospective observational cohort study reflecting the indications, dosing regimens, and side-effects of levosimendan, as well as patient outcomes over a year.
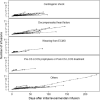
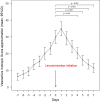
Results: The patients included (n = 602) represented 29.6% of the national yearly use of levosimendan in France. They were treated for cardiogenic shock (n = 250, 41.5%), decompensated heart failure (n = 127, 21.1%), cardiac surgery-related low cardiac output prophylaxis and/or treatment (n = 86, 14.3%), and weaning from veno-arterial extracorporeal membrane oxygenation (n = 82, 13.6%). They received 0.18 ± 0.07 µg/kg/min levosimendan over 26 ± 8 h. An initial bolus was administered in 45 patients (7.5%), 103 (17.1%) received repeated infusions, and 461 (76.6%) received inotropes and or vasoactive agents concomitantly. Hypotension was reported in 218 patients (36.2%), atrial fibrillation in 85 (14.1%), and serious adverse events in 17 (2.8%). 136 patients (22.6%) died in hospital, and 26 (4.3%) during the 90-day follow-up.
Conclusions: We observed that levosimendan was used in accordance with recent recommendations by French physicians. Hypotension and atrial fibrillation remained the most frequent side-effects, while serious adverse event potentially attributable to levosimendan were infrequent. The results suggest that this medication was safe and potentially associated with some benefit in the population studied.
Keywords: Cardiogenic shock; Heart failure; Inotropes; Levosimendan; Repetitive infusions; VA-ECMO weaning.
© 2023. La Société de Réanimation de Langue Francaise = The French Society of Intensive Care (SRLF).
Conflict of interest statement
Bernard Cholley, Bruno Levy, Alexandre Ouattara, Jean-Luc Fellahi, and Thibaut Caruba are part of an advisory board for Orion Pharma in charge of designing and coordinating the FRANCE LEVO registry, at the request of the French Haute Autorité de Santé. Philippe Mauriat is a consultant for Orion Pharma.
Figures


References
-
- Cholley B, Caruba T, Grosjean S, Amour J, Ouattara A, Villacorta J, et al. Effect of Levosimendan on low cardiac output syndrome in patients with low ejection fraction undergoing coronary artery bypass grafting with cardiopulmonary bypass: the LICORN randomized clinical trial. JAMA. 2017;318(6):548–556. doi: 10.1001/jama.2017.9973. - DOI - PMC - PubMed
-
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. - DOI - PubMed
LinkOut - more resources
Full Text Sources

