Evolutionary safety of lethal mutagenesis driven by antiviral treatment
- PMID: 37552682
- PMCID: PMC10409280
- DOI: 10.1371/journal.pbio.3002214
Evolutionary safety of lethal mutagenesis driven by antiviral treatment
Abstract
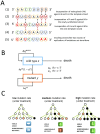
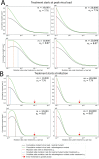
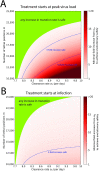
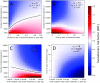
Nucleoside analogs are a major class of antiviral drugs. Some act by increasing the viral mutation rate causing lethal mutagenesis of the virus. Their mutagenic capacity, however, may lead to an evolutionary safety concern. We define evolutionary safety as a probabilistic assurance that the treatment will not generate an increased number of mutants. We develop a mathematical framework to estimate the total mutant load produced with and without mutagenic treatment. We predict rates of appearance of such virus mutants as a function of the timing of treatment and the immune competence of patients, employing realistic assumptions about the vulnerability of the viral genome and its potential to generate viable mutants. We focus on the case study of Molnupiravir, which is an FDA-approved treatment against Coronavirus Disease-2019 (COVID-19). We estimate that Molnupiravir is narrowly evolutionarily safe, subject to the current estimate of parameters. Evolutionary safety can be improved by restricting treatment with this drug to individuals with a low immunological clearance rate and, in future, by designing treatments that lead to a greater increase in mutation rate. We report a simple mathematical rule to determine the fold increase in mutation rate required to obtain evolutionary safety that is also applicable to other pathogen-treatment combinations.
Copyright: © 2023 Lobinska et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Khan S, Attar F, Bloukh SH, Sharifi M, Nabi F, Bai Q, et al. A review on the interaction of nucleoside analogues with SARS-CoV-2 RNA dependent RNA polymerase. International Journal of Biological Macromolecules. Int J Biol Macromol. 2021:605–611. doi: 10.1016/j.ijbiomac.2021.03.112 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

