Leak current, even with gigaohm seals, can cause misinterpretation of stem cell-derived cardiomyocyte action potential recordings
- PMID: 37552789
- PMCID: PMC10445319
- DOI: 10.1093/europace/euad243
Leak current, even with gigaohm seals, can cause misinterpretation of stem cell-derived cardiomyocyte action potential recordings
Abstract
Aims: Human-induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) have become an essential tool to study arrhythmia mechanisms. Much of the foundational work on these cells, as well as the computational models built from the resultant data, has overlooked the contribution of seal-leak current on the immature and heterogeneous phenotype that has come to define these cells. The aim of this study is to understand the effect of seal-leak current on recordings of action potential (AP) morphology.
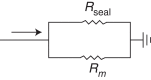
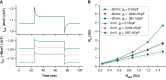
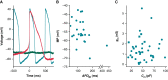
Methods and results: Action potentials were recorded in human iPSC-CMs using patch clamp and simulated using previously published mathematical models. Our in silico and in vitro studies demonstrate how seal-leak current depolarizes APs, substantially affecting their morphology, even with seal resistances (Rseal) above 1 GΩ. We show that compensation of this leak current is difficult due to challenges with obtaining accurate measures of Rseal during an experiment. Using simulation, we show that Rseal measures (i) change during an experiment, invalidating the use of pre-rupture values, and (ii) are polluted by the presence of transmembrane currents at every voltage. Finally, we posit that the background sodium current in baseline iPSC-CM models imitates the effects of seal-leak current and is increased to a level that masks the effects of seal-leak current on iPSC-CMs.
Conclusion: Based on these findings, we make recommendations to improve iPSC-CM AP data acquisition, interpretation, and model-building. Taking these recommendations into account will improve our understanding of iPSC-CM physiology and the descriptive ability of models built from such data.
Keywords: Arrhythmias; Computer simulation; Induced pluripotent stem cells; Ion channels; Patch clamp.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: T.P.B. is an Editorial Consultant of EP Europace and was not involved in the peer review process or publication decision. All remaining authors have declared no conflicts of interest.
Figures






Comment in
-
Mind the gap: leak currents and induced pluripotent stem cell-derived cardiomyocytes in translational cardiac electrophysiology.Europace. 2023 Aug 2;25(9):euad236. doi: 10.1093/europace/euad236. Europace. 2023. PMID: 37522360 Free PMC article.
Comment on
-
Low Resting Membrane Potential and Low Inward Rectifier Potassium Currents Are Not Inherent Features of hiPSC-Derived Cardiomyocytes.Stem Cell Reports. 2018 Mar 13;10(3):822-833. doi: 10.1016/j.stemcr.2018.01.012. Epub 2018 Feb 8. Stem Cell Reports. 2018. PMID: 29429959 Free PMC article.
-
The resting membrane potential of hSC-CM in a syncytium is more hyperpolarised than that of isolated cells.Channels (Austin). 2021 Dec;15(1):239-252. doi: 10.1080/19336950.2021.1871815. Channels (Austin). 2021. PMID: 33465001 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

