Convergent Deboronative and Decarboxylative Phosphonylation Enabled by the Phosphite Radical Trap "BecaP"
- PMID: 37552886
- PMCID: PMC10450818
- DOI: 10.1021/jacs.3c06524
Convergent Deboronative and Decarboxylative Phosphonylation Enabled by the Phosphite Radical Trap "BecaP"
Abstract
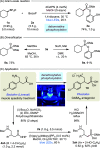
Carbon-phosphorus bond formation is significant in synthetic chemistry because phosphorus-containing compounds offer numerous indispensable biochemical roles. While there is a plethora of methods to access organophosphorus compounds, phosphonylations of readily accessible alkyl radicals to form aliphatic phosphonates are rare and not commonly used in synthesis. Herein, we introduce a novel phosphorus radical trap "BecaP" that enables facile and efficient phosphonylation of alkyl radicals under visible light photocatalytic conditions. Importantly, the ambiphilic nature of BecaP allows redox neutral reactions with both nucleophilic (activated by single-electron oxidation) and electrophilic (activated by single-electron reduction) alkyl radical precursors. Thus, a broad scope of feedstock alkyl potassium trifluoroborate salts and redox active carboxylate esters could be employed, with each class of substrate proceeding through a distinct mechanistic pathway. The mild conditions are applicable to the late-stage installation of phosphonate motifs into medicinal agents and natural products, which is showcased by the straightforward conversion of baclofen (muscle relaxant) to phaclofen (GABAB antagonist).
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Kolodiazhnyi O. I. Phosphorus Compounds of Natural Origin: Prebiotic, Stereochemistry, Application. Symmetry 2021, 13, 889. 10.3390/sym13050889. - DOI
- Moonen K.; Laureyn I.; Stevens C. V. Synthetic Methods for Azaheterocyclic Phosphonates and Their Biological Activity. Chem. Rev. 2004, 104, 6177–6216. 10.1021/cr030451c. - DOI - PubMed
- Papathanasiou K. E.; Vassaki M.; Spinthaki A.; Alatzoglou F.-E. G.; Tripodianos E.; Turhanen P.; Demadis K. D. Phosphorus Chemistry: From Small Molecules, to Polymers, to Pharmaceutical and Industrial applications. Pure Appl. Chem. 2019, 91, 421–441. 10.1515/pac-2018-1012. - DOI
-
- Rodriguez J. B.; Gallo-Rodriguez C. The Role of the Phosphorus Atom in Drug Design. ChemMedChem 2019, 14, 190–216. 10.1002/cmdc.201800693. - DOI - PubMed
- Yu H.; Yang H.; Shi E.; Tang W. Development and Clinical Application of Phosphorus-Containing Drugs. Med. Drug Discovery 2020, 8, 100063. 10.1016/j.medidd.2020.100063. - DOI - PMC - PubMed
-
- Diana G. D.; Zalay E. S.; Salvador U. J.; Pancic F.; Steinberg B. Synthesis of Some Phosphonates with Antiherpetic Activity. J. Med. Chem. 1984, 27, 691–694. 10.1021/jm00371a024. - DOI - PubMed
- Matsumoto T.; Nagata N.; Horikoshi N.; Αdachi I.; Ohashi Y.; Ogata E. Comparative Study of Incadronate and Elcatonin in Patients with Malignancy-Associated Hypercalcaemia. J. Int. Med. Res. 2002, 30, 230–243. 10.1177/147323000203000303. - DOI - PubMed
- Kerr D. I. B.; Ong J.; Prager R. H.; Gynther B. D.; Curtis D. R. Phaclofen: A Peripheral and Central Baclofen Antagonist. Brain Res. 1987, 405, 150–154. 10.1016/0006-8993(87)90999-1. - DOI - PubMed
- Evans R. H.; Francis A. A.; Jones A. W.; Smith D. A. S.; Watkins J. C. The Effects of a Series Of ω-Phosphonic α-Carboxylic Amino Acids on Electrically Evoked and Excitant Amino Acid-Induced Responses in Isolated Spinal Cord Preparations. Br. J. Pharmacol. 1982, 75, 65–75. 10.1111/j.1476-5381.1982.tb08758.x. - DOI - PMC - PubMed
- Behrendt C. T.; Kunfermann A.; Illarionova V.; Matheeussen A.; Pein M. K.; Gräwert T.; Kaiser J.; Bacher A.; Eisenreich W.; Illarionov B.; Fischer M.; Maes L.; Groll M.; Kurz T. Reverse Fosmidomycin Derivatives against the Antimalarial Drug Target IspC (Dxr). J. Med. Chem. 2011, 54, 6796–6802. 10.1021/jm200694q. - DOI - PubMed
- Zhang N.; Casida J. E. Novel Irreversible Butyrylcholinesterase Inhibitors: 2-Chloro-1-(substituted-phenyl)ethylphosphonic Acids. Bioorg. Med. Chem. 2002, 10, 1281–1290. 10.1016/s0968-0896(01)00391-1. - DOI - PubMed
-
- Montchamp J.-L. Phosphinate Chemistry in the 21st Century: A Viable Alternative to the Use of Phosphorus Trichloride in Organophosphorus Synthesis. Acc. Chem. Res. 2014, 47, 77–87. 10.1021/ar400071v. - DOI - PubMed
- Demmer C. S.; Krogsgaard-Larsen N.; Bunch L. Review on Modern Advances of Chemical Methods for the Introduction of a Phosphonic Acid Group. Chem. Rev. 2011, 111, 7981–8006. 10.1021/cr2002646. - DOI - PubMed
-
- Kostoudi S.; Pampalakis G. Improvements, Variations and Biomedical Applications of the Michaelis–Arbuzov Reaction. Int. J. Mol. Sci. 2022, 23, 3395. 10.3390/ijms23063395. - DOI - PMC - PubMed
- Oestreich M.; Tappe F.; Trepohl V. Transition-Metal-Catalyzed C–P Cross-Coupling Reactions. Synthesis 2010, 2010, 3037–3062. 10.1055/s-0030-1257960. - DOI
LinkOut - more resources
Full Text Sources

