mRNA vaccination boosts S-specific T cell memory and promotes expansion of CD45RAint TEMRA-like CD8+ T cells in COVID-19 recovered individuals
- PMID: 37552991
- PMCID: PMC10439252
- DOI: 10.1016/j.xcrm.2023.101149
mRNA vaccination boosts S-specific T cell memory and promotes expansion of CD45RAint TEMRA-like CD8+ T cells in COVID-19 recovered individuals
Abstract
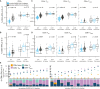
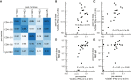
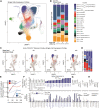
SARS-CoV-2 infection and mRNA vaccination both elicit spike (S)-specific T cell responses. To analyze how T cell memory from prior infection influences T cell responses to vaccination, we evaluated functional T cell responses in naive and previously infected vaccine recipients. Pre-vaccine S-specific responses are predictive of subsequent CD8+ T cell vaccine-response magnitudes. Comparing baseline with post-vaccination TCRβ repertoires, we observed large clonotypic expansions correlated with the frequency of spike-specific T cells. Epitope mapping the largest CD8+ T cell responses confirms that an HLA-A∗03:01 epitope was highly immunodominant. Peptide-MHC tetramer staining together with mass cytometry and single-cell sequencing permit detailed phenotyping and clonotypic tracking of these S-specific CD8+ T cells. Our results demonstrate that infection-induced S-specific CD8+ T cell memory plays a significant role in shaping the magnitude and clonal composition of the circulating T cell repertoire after vaccination, with mRNA vaccination promoting CD8+ memory T cells to a TEMRA-like phenotype.
Keywords: CD4 T cells; CD8 T cells; CyTOF; SARS-CoV-2; vaccination.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests E.W.N. is a cofounder, advisor, and shareholder of ImmunoScape and is an advisor for Neogene Therapeutics and NanoString Technologies.
Figures



References
-
- Kharbanda E.O., Vazquez-Benitez G. COVID-19 mRNA vaccines during pregnancy: New evidence to help address vaccine hesitancy. JAMA. 2022;327:1451–1453. - PubMed
-
- Tartof S.Y., Slezak J.M., Fischer H., Hong V., Ackerson B.K., Ranasinghe O.N., Frankland T.B., Ogun O.A., Zamparo J.M., Gray S., et al. Effectiveness of mRNA BNT162b2 COVID-19 vaccine up to 6 months in a large integrated health system in the USA: a retrospective cohort study. Lancet. 2021;398:1407–1416. - PMC - PubMed
-
- Arbel R., Sergienko R., Friger M., Peretz A., Beckenstein T., Yaron S., Netzer D., Hammerman A. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat. Med. 2022;28:1486–1490. doi: 10.1038/s41591-022-01832-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

