Adaptation versus plastic responses to temperature, light, and nitrate availability in cultured snow algal strains
- PMID: 37553143
- PMCID: PMC10481995
- DOI: 10.1093/femsec/fiad088
Adaptation versus plastic responses to temperature, light, and nitrate availability in cultured snow algal strains
Abstract
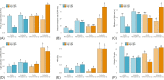
Snow algal blooms are widespread, dominating low temperature, high light, and oligotrophic melting snowpacks. Here, we assessed the photophysiological and cellular stoichiometric responses of snow algal genera Chloromonas spp. and Microglena spp. in their vegetative life stage isolated from the Arctic and Antarctic to gradients in temperature (5 - 15°C), nitrate availability (1 - 10 µmol L-1), and light (50 and 500 µmol photons m-2 s-1). When grown under gradients in temperature, measured snow algal strains displayed Fv/Fm values increased by ∼115% and electron transport rates decreased by ∼50% at 5°C compared to 10 and 15°C, demonstrating how low temperatures can mimic high light impacts to photophysiology. When using carrying capacity as opposed to growth rate as a metric for determining the temperature optima, these snow algal strains can be defined as psychrophilic, with carrying capacities ∼90% higher at 5°C than warmer temperatures. All strains approached Redfield C:N stoichiometry when cultured under nutrient replete conditions regardless of temperature (5.7 ± 0.4 across all strains), whereas significant increases in C:N were apparent when strains were cultured under nitrate concentrations that reflected in situ conditions (17.8 ± 5.9). Intra-specific responses in photophysiology were apparent under high light with Chloromonas spp. more capable of acclimating to higher light intensities. These findings suggest that in situ conditions are not optimal for the studied snow algal strains, but they are able to dynamically adjust both their photochemistry and stoichiometry to acclimate to these conditions.
Keywords: photophysiology; psychrophile; snow algae; stoichiometry.
© The Author(s) 2023. Published by Oxford University Press on behalf of FEMS.
Conflict of interest statement
None declared.
Figures



References
-
- Andersen RA. Algal Culturing Techniques. Cambridge: Academic Press, 2005.
-
- Anesio AM, Laybourn-Parry J. Glaciers and ice sheets as a biome. Trends Ecol Evol. 2012;27:219–25. - PubMed
-
- Arteaga L, Pahlow M, Oschlies A. Global patterns of phytoplankton nutrient and light colimitation inferred from an optimality-based model. Glob Biogeochem Cycl. 2014;28:648–61.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

