IKBKB reduces huntingtin aggregation by phosphorylating serine 13 via a non-canonical IKK pathway
- PMID: 37553253
- PMCID: PMC10410066
- DOI: 10.26508/lsa.202302006
IKBKB reduces huntingtin aggregation by phosphorylating serine 13 via a non-canonical IKK pathway
Abstract
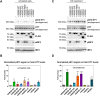
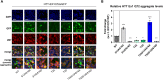
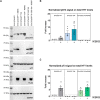
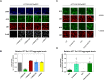
N-terminal phosphorylation at residues T3 and S13 is believed to have important beneficial implications for the biological and pathological properties of mutant huntingtin, where inhibitor of nuclear factor kappa B kinase subunit beta (IKBKB) was identified as a candidate regulator of huntingtin N-terminal phosphorylation. The paucity of mechanistic information on IKK pathways, together with the lack of sensitive methods to quantify endogenous huntingtin phosphorylation, prevented detailed study of the role of IKBKB in Huntington's disease. Using novel ultrasensitive assays, we demonstrate that IKBKB can regulate endogenous S13 huntingtin phosphorylation in a manner, dependent on its kinase activity and known regulators. We found that the ability of IKBKB to phosphorylate endogenous huntingtin S13 is mediated through a non-canonical interferon regulatory factor3-mediated IKK pathway, distinct from the established involvement of IKBKB in mutant huntingtin's pathological mechanisms mediated via the canonical pathway. Furthermore, increased huntingtin S13 phosphorylation by IKBKB resulted in decreased aggregation of mutant huntingtin in cells, again dependent on its kinase activity. These findings point to a non-canonical IKK pathway linking S13 huntingtin phosphorylation to the pathological properties of mutant huntingtin aggregation, thought to be significant to Huntington's disease.
© 2023 Cariulo et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures





References
-
- Ansaloni A, Wang ZM, Jeong JS, Ruggeri FS, Dietler G, Lashuel HA (2014) One-pot semisynthesis of exon 1 of the huntingtin protein: New tools for elucidating the role of posttranslational modifications in the pathogenesis of Huntington's disease. Angew Chem Int Ed Engl 53: 1928–1933. 10.1002/anie.201307510 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
