Long-term ex situ normothermic perfusion of human split livers for more than 1 week
- PMID: 37553343
- PMCID: PMC10409852
- DOI: 10.1038/s41467-023-40154-8
Long-term ex situ normothermic perfusion of human split livers for more than 1 week
Abstract
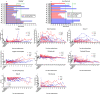
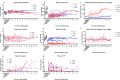
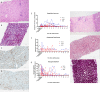
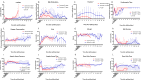
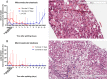
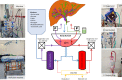
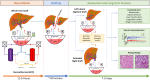
Current machine perfusion technology permits livers to be preserved ex situ for short periods to assess viability prior to transplant. Long-term normothermic perfusion of livers is an emerging field with tremendous potential for the assessment, recovery, and modification of organs. In this study, we aimed to develop a long-term model of ex situ perfusion including a surgical split and simultaneous perfusion of both partial organs. Human livers declined for transplantation were perfused using a red blood cell-based perfusate under normothermic conditions (36 °C) and then split and simultaneously perfused on separate machines. Ten human livers were split, resulting in 20 partial livers. The median ex situ viability was 125 h, and the median ex situ survival was 165 h. Long-term survival was demonstrated by lactate clearance, bile production, Factor-V production, and storage of adenosine triphosphate. Here, we report the long-term ex situ perfusion of human livers and demonstrate the ability to split and perfuse these organs using a standardised protocol.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

