Synthesis of a novel multifunctional organic-inorganic nanocomposite for metal ions and organic dye removals
- PMID: 37553434
- PMCID: PMC10409728
- DOI: 10.1038/s41598-023-38420-2
Synthesis of a novel multifunctional organic-inorganic nanocomposite for metal ions and organic dye removals
Abstract
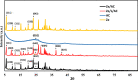
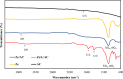
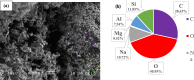
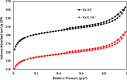
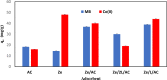
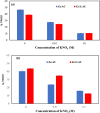
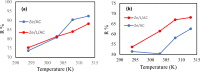
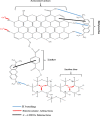
In this study, we used solvent assisted mechano-synthesis strategies to form multifunctional organic-inorganic nanocomposites capable of removing both organic and inorganic contaminants. A zeolite X (Ze) and activated carbon (AC) composite was synthesized via state-of-the-art mechanical mixing in the presence of few drops of water to form Ze/AC. The second composite (Ze/L/AC) was synthesized in a similar fashion, however this composite had the addition of disodium terephthalate as a linker. Both materials, Ze/AC and Ze/L/AC, were characterized using scanning electron microscope (SEM), energy-dispersive X-ray spectroscopy (EDS), Powdered X-ray diffraction (P-XRD), Fourier-transform infrared spectrometry (FTIR), Accelerated Surface Area and Porosimetry System (ASAP), and thermal gravimetric analysis (TGA). The SEM-EDS displayed the surface structure and composition of each material. The sodium, oxygen and carbon contents increased after linker connected Ze and AC. The P-XRD confirmed the crystallinity of each material as well as the composites, while FTIR indicated the function groups (C=C, O-H) in Ze/L/AC. The contaminant adsorption experiments investigated the effects of pH, temperature, and ionic strength on the adsorption of methylene blue (MB) and Co(II) for each material. In MB adsorption, the first-order reaction rate of Ze/L/AC (0.02 h-1) was double that of Ze/AC (0.01 h-1). The reaction rate of Ze/L/AC (4.8 h-1) was also extraordinarily higher than that of Ze/AC (0.6 h-1) in the adsorption of Co(II). Ze/L/AC composite achieved a maximum adsorption capacity of 44.8 mg/g for MB and 66.6 mg/g for Co(II) ions. The MB adsorption of Ze/AC and Ze/L/AC was best fit in Freundlich model with R2 of 0.96 and 0.97, respectively, which indicated the multilayer adsorption. In the Co(II) adsorption, the data was highly fit in Langmuir model with R2 of 0.94 and 0.92 which indicated the monolayer adsorption. These results indicated both materials exhibited chemisorption. The activation energy of Ze/L/AC in MB adsorption (34.9 kJ mol-1) was higher than that of Ze/L/AC in Co (II) adsorption (26 kJ mol-1).
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Vardhan KH, Kumar PS, Panda RC. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019;290:111197. doi: 10.1016/j.molliq.2019.111197. - DOI
-
- Rad LR, Anbia M. Zeolite-based composites for the adsorption of toxic matters from water: A review. J. Environ. Chem. Eng. 2021;9:106088. doi: 10.1016/j.jece.2021.106088. - DOI
-
- International Agency for Research on Cancer. Arsenic and arsenic compounds. In: IARC IARC Monogr Eval Carcinog Risks to Humans-Overall Eval Carcinog an Updat IARC Mongraphs 100 (1987)
LinkOut - more resources
Full Text Sources
Miscellaneous

