Macrophage-organoid co-culture model for identifying treatment strategies against macrophage-related gemcitabine resistance
- PMID: 37553567
- PMCID: PMC10411021
- DOI: 10.1186/s13046-023-02756-4
Macrophage-organoid co-culture model for identifying treatment strategies against macrophage-related gemcitabine resistance
Abstract
Background: Gemcitabine resistance (GR) is a significant clinical challenge in pancreatic adenocarcinoma (PAAD) treatment. Macrophages in the tumor immune-microenvironment are closely related to GR. Uncovering the macrophage-induced GR mechanism could help devise a novel strategy to improve gemcitabine treatment outcomes in PAAD. Therefore, preclinical models accurately replicating patient tumor properties are essential for cancer research and drug development. Patient-derived organoids (PDOs) represent a promising in vitro model for investigating tumor targets, accelerating drug development, and enabling personalized treatment strategies to improve patient outcomes.
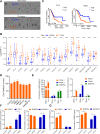
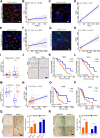
Methods: To investigate the effects of macrophage stimulation on GR, co-cultures were set up using PDOs from three PAAD patients with macrophages. To identify signaling factors between macrophages and pancreatic cancer cells (PCCs), a 97-target cytokine array and the TCGA-GTEx database were utilized. The analysis revealed CCL5 and AREG as potential candidates. The role of CCL5 in inducing GR was further investigated using clinical data and tumor sections obtained from 48 PAAD patients over three years, inhibitors, and short hairpin RNA (shRNA). Furthermore, single-cell sequencing data from the GEO database were analyzed to explore the crosstalk between PCCs and macrophages. To overcome GR, inhibitors targeting the macrophage-CCL5-Sp1-AREG feedback loop were evaluated in cell lines, PDOs, and orthotopic mouse models of pancreatic carcinoma.
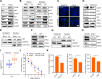
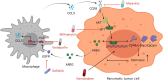
Results: The macrophage-CCL5-Sp1-AREG feedback loop between macrophages and PCCs is responsible for GR. Macrophage-derived CCL5 activates the CCR5/AKT/Sp1/CD44 axis to confer stemness and chemoresistance to PCCs. PCC-derived AREG promotes CCL5 secretion in macrophages through the Hippo-YAP pathway. By targeting the feedback loop, mithramycin improves the outcome of gemcitabine treatment in PAAD. The results from the PDO model were corroborated with cell lines, mouse models, and clinical data.
Conclusions: Our study highlights that the PDO model is a superior choice for preclinical research and precision medicine. The macrophage-CCL5-Sp1-AREG feedback loop confers stemness to PCCs to facilitate gemcitabine resistance by activating the CCR5/AKT/SP1/CD44 pathway. The combination of gemcitabine and mithramycin shows potential as a therapeutic strategy for treating PAAD in cell lines, PDOs, and mouse models.
Keywords: Cancer stem cells; Gemcitabine resistance; Macrophages; Organoids.
© 2023. Italian National Cancer Institute ‘Regina Elena’.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Yang J, Liao D, Chen C, Liu Y, Chuang TH, Xiang R, et al. Tumor-associated macrophages regulate murine breast cancer stem cells through a novel paracrine EGFR/Stat3/Sox-2 signaling pathway. Stem cells. 2013;31:248–258. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

