Hijacking homeostasis: Regulation of the tumor microenvironment by apoptosis
- PMID: 37553811
- PMCID: PMC10952466
- DOI: 10.1111/imr.13259
Hijacking homeostasis: Regulation of the tumor microenvironment by apoptosis
Abstract
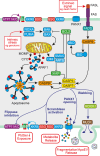
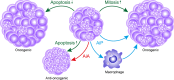
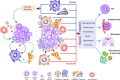
Cancers are genetically driven, rogue tissues which generate dysfunctional, obdurate organs by hijacking normal, homeostatic programs. Apoptosis is an evolutionarily conserved regulated cell death program and a profoundly important homeostatic mechanism that is common (alongside tumor cell proliferation) in actively growing cancers, as well as in tumors responding to cytotoxic anti-cancer therapies. Although well known for its cell-autonomous tumor-suppressive qualities, apoptosis harbors pro-oncogenic properties which are deployed through non-cell-autonomous mechanisms and which generally remain poorly defined. Here, the roles of apoptosis in tumor biology are reviewed, with particular focus on the secreted and fragmentation products of apoptotic tumor cells and their effects on tumor-associated macrophages, key supportive cells in the aberrant homeostasis of the tumor microenvironment. Historical aspects of cell loss in tumor growth kinetics are considered and the impact (and potential impact) on tumor growth of apoptotic-cell clearance (efferocytosis) as well as released soluble and extracellular vesicle-associated factors are discussed from the perspectives of inflammation, tissue repair, and regeneration programs. An "apoptosis-centric" view is proposed in which dying tumor cells provide an important platform for intricate intercellular communication networks in growing cancers. The perspective has implications for future research and for improving cancer diagnosis and therapy.
Keywords: apoptosis; cancer; efferocytosis; extracellular vesicle; homeostasis; macrophage; tumor microenvironment.
© 2023 The Author. Immunological Reviews published by John Wiley & Sons Ltd.
Conflict of interest statement
The author declares no conflict of interest.
Figures

Similar articles
-
Efferocytosis and the Story of "Find Me," "Eat Me," and "Don't Eat Me" Signaling in the Tumor Microenvironment.Adv Exp Med Biol. 2021;1329:153-162. doi: 10.1007/978-3-030-73119-9_8. Adv Exp Med Biol. 2021. PMID: 34664238
-
Efferocytosis in the tumor microenvironment.Semin Immunopathol. 2018 Nov;40(6):545-554. doi: 10.1007/s00281-018-0698-5. Epub 2018 Sep 5. Semin Immunopathol. 2018. PMID: 30187085 Free PMC article. Review.
-
Assessment of the Immune Response to Tumor Cell Apoptosis and Efferocytosis.Methods Mol Biol. 2022;2543:45-55. doi: 10.1007/978-1-0716-2553-8_5. Methods Mol Biol. 2022. PMID: 36087258
-
An apoptosis-driven 'onco-regenerative niche': roles of tumour-associated macrophages and extracellular vesicles.Philos Trans R Soc Lond B Biol Sci. 2018 Jan 5;373(1737):20170003. doi: 10.1098/rstb.2017.0003. Philos Trans R Soc Lond B Biol Sci. 2018. PMID: 29158317 Free PMC article. Review.
-
Treatment-Induced Tumor Cell Apoptosis and Secondary Necrosis Drive Tumor Progression in the Residual Tumor Microenvironment through MerTK and IDO1.Cancer Res. 2019 Jan 1;79(1):171-182. doi: 10.1158/0008-5472.CAN-18-1106. Epub 2018 Nov 9. Cancer Res. 2019. PMID: 30413412
Cited by
-
C-reactive protein-induced injury in Mycoplasma pneumoniae-infected lung epithelial cells is mediated by the P38 MAPK/mitochondrial apoptosis pathway.Microbiol Spectr. 2025 Mar 4;13(3):e0162624. doi: 10.1128/spectrum.01626-24. Epub 2025 Feb 11. Microbiol Spectr. 2025. PMID: 39932324 Free PMC article.
-
OGG1 as an Epigenetic Reader Affects NFκB: What This Means for Cancer.Cancers (Basel). 2023 Dec 28;16(1):148. doi: 10.3390/cancers16010148. Cancers (Basel). 2023. PMID: 38201575 Free PMC article. Review.
-
Contribution of tumor microenvironment (TME) to tumor apoptosis, angiogenesis, metastasis, and drug resistance.Med Oncol. 2025 Mar 14;42(4):108. doi: 10.1007/s12032-025-02675-8. Med Oncol. 2025. PMID: 40087196 Review.
-
Mechanism of Efferocytosis in Determining Ischaemic Stroke Resolution-Diving into Microglia/Macrophage Functions and Therapeutic Modality.Mol Neurobiol. 2024 Oct;61(10):7583-7602. doi: 10.1007/s12035-024-04060-4. Epub 2024 Feb 27. Mol Neurobiol. 2024. PMID: 38409642 Review.
-
Apoptosis-targeting BH3 mimetics: transforming treatment for patients with acute myeloid leukaemia.Nat Rev Clin Oncol. 2025 Sep 1. doi: 10.1038/s41571-025-01068-0. Online ahead of print. Nat Rev Clin Oncol. 2025. PMID: 40890352 Review.
References
-
- WHO . The top 10 causes of death . 2020. https://www.who.int/news‐room/fact‐sheets/detail/the‐top‐10‐causes‐of‐de...
-
- Steel GG. Growth Kinetics of Tumours: Cell Population Kinetics in Relation to the Growth and Treatment of Cancer. Clarendon Press; 1977.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

