Synthesis, stereochemical assignment, and enantioselective catalytic activity of late transition metal planar chiral complexes
- PMID: 37554058
- PMCID: PMC10507873
- DOI: 10.1039/d3cs00325f
Synthesis, stereochemical assignment, and enantioselective catalytic activity of late transition metal planar chiral complexes
Abstract
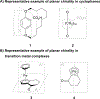
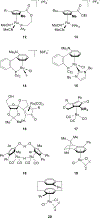
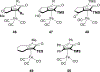
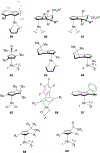
Planar chirality is an important form of molecular chirality that can be utilized to induce enantioselectivity when incorporated into transition metal catalysts. However, due to synthetic constraints, the use of late transition metal planar chiral complexes to conduct enantioselective transformations has been limited. Additionally, the published methods surrounding the stereochemical assignment of planar chiral compounds are sometimes conflicting, making proper assignment difficult. This review aims to provide clarity on the methods available to assign planar chirality and provide an overview on the synthesis and use of late transition metal planar chiral complexes as enantioselective catalysts.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures



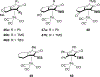
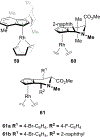

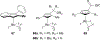
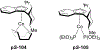
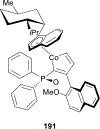



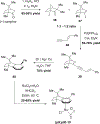
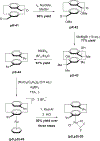
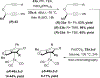

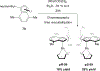
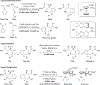
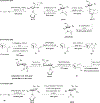
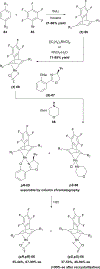
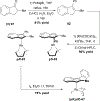
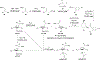
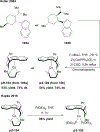
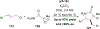

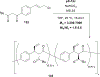

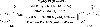
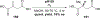
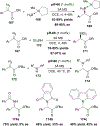
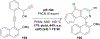
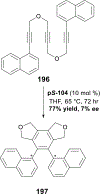
References
-
- Mas-Roselló J, Herraiz AG, Audic B, Laverny A and Cramer N, Angew. Chem. Int. Ed, 2020, 60, 13198–13224. - PubMed
-
- Jia Z-J, Merten C, Gontla R, Daniliuc CG, Antonchick AP and Waldmann H, Angew. Chem. Int. Ed, 2017, 56, 2429–2434. - PubMed
-
- Cui WJ, Wu ZJ, Gu Q and You SL, J. Am. Chem. Soc, 2020, 142, 7379–7385. - PubMed
-
- Farr CMB, Kazerouni AM, Park B, Poff CD, Won J, Sharp KR, Baik MH and Blakey SB, J. Am. Chem. Soc, 2020, 142, 13996–14004. - PubMed
-
- Kataoka Y, Iwato Y, Yamagata T and Tani K, Organometallics, 1999, 18, 5423–5425.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

