Genome-wide impact of cytosine methylation and DNA sequence context on UV-induced CPD formation
- PMID: 37554110
- PMCID: PMC10853481
- DOI: 10.1002/em.22569
Genome-wide impact of cytosine methylation and DNA sequence context on UV-induced CPD formation
Abstract
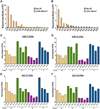
Exposure to ultraviolet (UV) light is the primary etiological agent for skin cancers because UV damages cellular DNA. The most frequent form of UV damage is the cyclobutane pyrimidine dimer (CPD), which consists of covalent linkages between neighboring pyrimidine bases in DNA. In human cells, the 5' position of cytosine bases in CG dinucleotides is frequently methylated, and methylated cytosines in the TP53 tumor suppressor are often sites of mutation hotspots in skin cancers. It has been argued that this is because cytosine methylation promotes UV-induced CPD formation; however, the effects of cytosine methylation on CPD formation are controversial, with conflicting results from previous studies. Here, we use a genome-wide method known as CPD-seq to map UVB- and UVC-induced CPDs across the yeast genome in the presence or absence in vitro methylation by the CpG methyltransferase M.SssI. Our data indicate that cytosine methylation increases UVB-induced CPD formation nearly 2-fold relative to unmethylated DNA, but the magnitude of induction depends on the flanking sequence context. Sequence contexts with a 5' guanine base (e.g., GCCG and GTCG) show the strongest induction due to cytosine methylation, potentially because these sequence contexts are less efficient at forming CPD lesions in the absence of methylation. We show that cytosine methylation also modulates UVC-induced CPD formation, albeit to a lesser extent than UVB. These findings can potentially reconcile previous studies, and define the impact of cytosine methylation on UV damage across a eukaryotic genome.
Keywords: 5‐methylcytosine; DNA sequence specificity; UV‐induced damage; cyclobutane pyrimidine dimers (CPDs); genome‐wide damage mapping.
© 2023 The Authors. Environmental and Molecular Mutagenesis published by Wiley Periodicals LLC on behalf of Environmental Mutagenesis and Genomics Society.
Figures

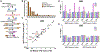
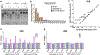


References
-
- Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjord JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinsk M, Jager N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, Lopez-Otin C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdes-Mas R, van Buuren MM, van 't Veer L, Vincent-Salomon A, Waddell N, Yates LR, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ and Stratton MR (2013) Signatures of mutational processes in human cancer. Nature 500:415–21. - PMC - PubMed
-
- Banyasz A, Esposito L, Douki T, Perron M, Lepori C, Improta R and Markovitsi D (2016) Effect of C5-Methylation of Cytosine on the UV-Induced Reactivity of Duplex DNA: Conformational and Electronic Factors. J Phys Chem B 120:4232–42. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

