Impact of interval progression before autologous stem cell transplant in patients with multiple myeloma
- PMID: 37554170
- PMCID: PMC10405820
- DOI: 10.3389/fonc.2023.1216461
Impact of interval progression before autologous stem cell transplant in patients with multiple myeloma
Abstract
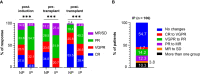
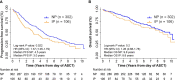
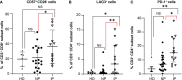
In transplant-eligible patients who undergo upfront autologous stem cell transplant (ASCT) for multiple myeloma (MM), standard practice is to treat with six to eight cycles of induction therapy followed by high-dose chemotherapy with ASCT. A gap between the end of induction and the day of ASCT exists to allow stem cell mobilization and collection. Despite attempts to limit the length of this interval, we noticed that some patients experience interval progression (IP) of disease between the end of induction therapy and the day of ASCT. We analyzed 408 MM patients who underwent ASCT between 2011 and 2016. The median length of the interval between end of induction and ASCT was 38 days. We observed that 26% of patients in the entire cohort and 23.6% of patients who received induction with bortezomib-lenalidomide-dexamethasone (VRD) experienced IP. These patients deepened their responses with ASCT, independently of induction regimen. In the entire cohort, IP was significantly associated with shorter PFS in the univariable analysis (Hazard Ratio, HR = 1.37, P = 0.022) but not in the multivariable analysis (HR = 1.14, P = 0.44). However, analyzing only patients who received VRD as induction, progression-free survival (PFS) remained inferior in both the univariable (HR = 2.02; P = 0.002) and the multivariable analyses (HR = 1.96; P = 0.01). T cells and natural killer (NK) cells are increasingly studied targets of immunomodulatory therapy, as immune dysfunction is known to occur in patients with MM. Peripheral blood from 35 MM patients were analyzed. At time of ASCT, patients with IP had significantly increased percentages of CD3+CD8+CD57+ CD28- (P = 0.05) and CD3+CD4+LAG3+ (P = 0.0022) T-cells, as well as less CD56bright and CD56dim NK cells bearing activated markers such as CD69, NKG2D, and CD226. These data suggest that IP can impact the length of response to ASCT; therefore, further studies on the management of these patients are needed.
Keywords: disease progression; immune profiling; myeloma; outcome; stem cell transplant (SCT).
Copyright © 2023 Bao, Zhao, Kudalkar, Rodriguez, Sharma, Bumma, Devarakonda, Khan, Umyarova, Rosko, Benson and Cottini.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

