iNOS aggravates pressure overload-induced cardiac dysfunction via activation of the cytosolic-mtDNA-mediated cGAS-STING pathway
- PMID: 37554263
- PMCID: PMC10405855
- DOI: 10.7150/thno.84049
iNOS aggravates pressure overload-induced cardiac dysfunction via activation of the cytosolic-mtDNA-mediated cGAS-STING pathway
Abstract
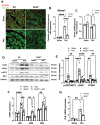
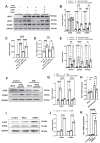
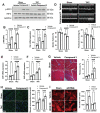
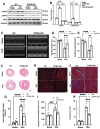
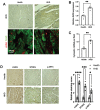
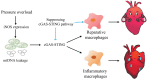
Background: Sterile inflammation contributes to the pathogenesis of cardiac dysfunction caused by various conditions including pressure overload in hypertension. Mitochondrial DNA (mtDNA) released from damaged mitochondria has been implicated in cardiac inflammation. However, the upstream mechanisms governing mtDNA release and how mtDNA activates sterile inflammation in pressure-overloaded hearts remain largely unknown. Here, we investigated the role of inducible NO synthase (iNOS) on pressure overload-induced cytosolic accumulation of mtDNA and whether mtDNA activated inflammation through the cyclic GMP-AMP synthase (cGAS)-stimulator of interferon genes (STING) pathway. Methods: To investigate whether the cGAS-STING cascade was involved in sterile inflammation and cardiac dysfunction upon pressure overload, cardiomyocyte-specific STING depletion mice and mice injected with adeno-associated virus-9 (AAV-9) to suppress the cGAS-STING cascade in the heart were subjected to transverse aortic constriction (TAC). iNOS null mice were used to determine the role of iNOS in cGAS-STING pathway activation in pressure-stressed hearts. Results: iNOS knockout abrogated mtDNA release and alleviated cardiac sterile inflammation resulting in improved cardiac function. Conversely, activating the cGAS-STING pathway blunted the protective effects of iNOS knockout. Moreover, iNOS activated the cGAS-STING pathway in isolated myocytes and this was prevented by depleting cytosolic mtDNA. In addition, disruption of the cGAS-STING pathway suppressed inflammatory cytokine transcription and modulated M1/M2 macrophage polarization, and thus mitigated cardiac remodeling and improved heart function. Finally, increased iNOS expression along with cytosolic mtDNA accumulation and cGAS-STING activation were also seen in human hypertensive hearts. Conclusion: Our findings demonstrate that mtDNA is released into the cytosol and triggers sterile inflammation through the cGAS-STING pathway leading to cardiac dysfunction after pressure overload. iNOS controls mtDNA release and subsequent cGAS activation in pressure-stressed hearts.
Keywords: Cardiac dysfunction; Sterile inflammation; cGAS; iNOS; mtDNA.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Fujiu K, Nagai R. Contributions of cardiomyocyte-cardiac fibroblast-immune cell interactions in heart failure development. Basic Res Cardiol. 2013;108:357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

