Click-to-Release: Cleavable Radioimmunoimaging with [89Zr]Zr-DFO- Trans-Cyclooctene-Trastuzumab Increases Tumor-to-Blood Ratio
- PMID: 37554267
- PMCID: PMC10405837
- DOI: 10.7150/thno.84865
Click-to-Release: Cleavable Radioimmunoimaging with [89Zr]Zr-DFO- Trans-Cyclooctene-Trastuzumab Increases Tumor-to-Blood Ratio
Abstract
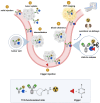
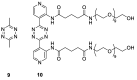
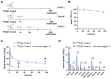
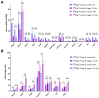
One of the main challenges of PET imaging with 89Zr-labeled monoclonal antibodies (mAbs) remains the long blood circulation of the radiolabeled mAbs, leading to high background signals, decreasing image quality. To overcome this limitation, here we report the use of a bioorthogonal linker cleavage approach (click-to-release chemistry) to selectively liberate [89Zr]Zr-DFO from trans-cyclooctene-functionalized trastuzumab (TCO-Tmab) in blood, following the administration of a tetrazine compound (trigger) in BT-474 tumor-bearing mice. Methods: We created a series of TCO-DFO constructs and evaluated their performance in [89Zr]Zr-DFO release from Tmab in vitro using different trigger compounds. The in vivo behavior of the best performing [89Zr]Zr-TCO-Tmab was studied in healthy mice first to determine the optimal dose of the trigger. To find the optimal time for the trigger administration, the rate of [89Zr]Zr-TCO-Tmab internalization was studied in BT-474 cancer cells. Finally, the trigger was administered 6 h or 24 h after [89Zr]Zr-TCO-Tmab- administration in tumor-bearing mice to liberate the [89Zr]Zr-DFO fragment. PET scans were obtained of tumor-bearing mice that received the trigger 6 h post-[89Zr]Zr-TCO-Tmab administration. Results: The [89Zr]Zr-TCO-Tmab and trigger pair with the best in vivo properties exhibited 83% release in 50% mouse plasma. In tumor-bearing mice the tumor-blood ratios were markedly increased from 1.0 ± 0.4 to 2.3 ± 0.6 (p = 0.0057) and from 2.5 ± 0.7 to 6.6 ± 0.9 (p < 0.0001) when the trigger was administered at 6 h and 24 h post-mAb, respectively. Same day PET imaging clearly showed uptake in the tumor combined with a strongly reduced background due to the fast clearance of the released [89Zr]Zr-DFO-containing fragment from the circulation through the kidneys. Conclusions: This is the first demonstration of the use of trans-cyclooctene-tetrazine click-to-release chemistry to release a radioactive chelator from a mAb in mice to increase tumor-to-blood ratios. Our results suggest that click-cleavable radioimmunoimaging may allow for substantially shorter intervals in PET imaging with full mAbs, reducing radiation doses and potentially even enabling same day imaging.
Keywords: IEDDA; click-to-release; radioimmunoimaging; trans-cyclooctene; trastuzumab.
© The author(s).
Conflict of interest statement
Competing Interests: Maria Vlastara, Raffaella Rossin, Kim de Roode, Laurens Kleijn, and Marc Robillard are employees and/or shareholders of Tagworks. The other authors have no competing interests to declare.
Figures




References
-
- Posner J, Barrington P, Brier T, Datta-Mannan A. Monoclonal antibodies: past, present and future. Concepts and Principles of Pharmacology: 100 Years of the Handbook of Experimental Pharmacology. 2019;260:81–141. - PubMed
-
- Lin M, Paolillo V, Le DB, Macapinlac H, Ravizzini GC. Monoclonal antibody based radiopharmaceuticals for imaging and therapy. Curr Probl Canc. 2021;45:100796. - PubMed
-
- Dilworth JR, Pascu SI. The chemistry of PET imaging with zirconium-89. Chem Soc Rev. 2018;47:2554–71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

