Recent advances in endogenous neural stem/progenitor cell manipulation for spinal cord injury repair
- PMID: 37554275
- PMCID: PMC10405838
- DOI: 10.7150/thno.84133
Recent advances in endogenous neural stem/progenitor cell manipulation for spinal cord injury repair
Abstract
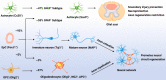
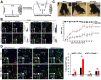
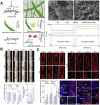
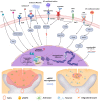
Traumatic spinal cord injury (SCI) can cause severe neurological impairments. Clinically available treatments are quite limited, with unsatisfactory remediation effects. Residing endogenous neural stem/progenitor cells (eNSPCs) tend to differentiate towards astrocytes, leaving only a small fraction towards oligodendrocytes and even fewer towards neurons; this has been suggested as one of the reasons for the failure of autonomous neuronal regeneration. Thus, finding ways to recruit and facilitate the differentiation of eNSPCs towards neurons has been considered a promising strategy for the noninvasive and immune-compatible treatment of SCI. The present manuscript first introduces the responses of eNSPCs after exogenous interventions to boost endogenous neurogenesis in various SCI models. Then, we focus on state-of-art manipulation approaches that enhance the intrinsic neurogenesis capacity and reconstruct the hostile microenvironment, mainly consisting of pharmacological treatments, stem cell-derived exosome administration, gene therapy, functional scaffold implantation, inflammation regulation, and inhibitory element delineation. Facing the extremely complex situation of SCI, combined treatments are also highlighted to provide more clues for future relevant investigations.
Keywords: Endogenous neural stem/progenitor cells; combined treatment; neurogenesis; spinal cord injury repair.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
References
-
- Eckert MJ, Martin MJ. Trauma: Spinal Cord Injury. Surg Clin North Am. 2017;97:1031–45. - PubMed
-
- Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: update 2011, global incidence rate. Spinal Cord. 2014;52:110–6. - PubMed
-
- Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A. et al. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

