STING-IRG1 inhibits liver metastasis of colorectal cancer by regulating the polarization of tumor-associated macrophages
- PMID: 37554436
- PMCID: PMC10405073
- DOI: 10.1016/j.isci.2023.107376
STING-IRG1 inhibits liver metastasis of colorectal cancer by regulating the polarization of tumor-associated macrophages
Abstract
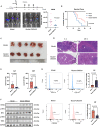
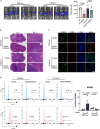
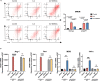
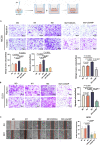
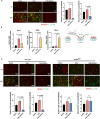
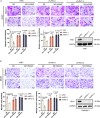
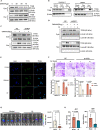
The liver is the main site of colorectal cancer (CRC) metastasis. Tumor-associated macrophages (TAMs) play a key role in tumor metastasis. Therefore, modulating the function of tumor-associated macrophages is a potential therapeutic strategy to control tumor metastasis. We found in vivo experiments that the activation of STING inhibited CRC liver metastasis in model mice and affected the macrophage phenotype in the tumor microenvironment. Mechanistically, STING affects TAM polarization and regulates macrophage function through IRG1. And STING activates IRG1 to promote the nuclear translocation of TFEB, affecting the ability of macrophages to suppress tumor metastasis.Therefore, this study highlights the critical role of the STING-IRG1 axis on TAM reprogramming and its role in the process of tumor liver metastasis, which may provide a promising therapeutic strategy for CRC liver metastasis.
Keywords: Biological sciences; Immunity; cancer; molecular biology.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

