Roquin-1 resolves sepsis-associated acute liver injury by regulating inflammatory profiles via miRNA cargo in extracellular vesicles
- PMID: 37554446
- PMCID: PMC10405074
- DOI: 10.1016/j.isci.2023.107295
Roquin-1 resolves sepsis-associated acute liver injury by regulating inflammatory profiles via miRNA cargo in extracellular vesicles
Abstract
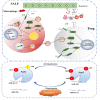
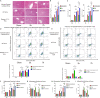
Sepsis-associated acute liver injury (SALI) is an independent risk for sepsis-induced death orchestrated by innate and adaptive immune responses. Here, we found that Roquin-1 was decreased during SALI and expressed mainly in monocyte-derived macrophages. Meanwhile, Roquin-1 was correlated with the inflammatory profiles in humans and mice. Mechanically, Roquin-1 in macrophages promoted Ago2-K258-ubiquitination and inhibited Ago2-S387/S828-phosphorylation. Ago2-S387-phosphorylation inhibited Ago2-miRNA's complex location in multivesicular bodies and sorting in macrophages-derived extracellular vesicles (MDEVs), while Ago2-S828-phosphorylation modulated the binding between Ago2 and miRNAs by special miRNAs-motifs. Then, the anti-inflammatory miRNAs in MDEVs decreased TSC22D2 expression directly, upregulated Tregs-differentiation via TSC22D2-STAT3 signaling, and inhibited M1-macrophage-polarization by TSC22D2-AMPKα-mTOR pathway. Furthermore, WT MDEVs in mice alleviated SALI by increasing Tregs ratio and decreasing M1-macrophage frequency synchronously. Our study showed that Roquin-1 in macrophages increased Tregs-differentiation and decreased M1-macrophage-polarization simultaneously via miRNA in MDEVs, suggesting Roquin-1 can be used as a potential tool for SALI treatment and MDEVs engineering.
Keywords: Immunology; Molecular biology; Pathophysiology.
© 2023.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures







References
LinkOut - more resources
Full Text Sources
Miscellaneous

