Skin Infiltrate Composition as a Telling Measure of Responses to Checkpoint Inhibitors
- PMID: 37554516
- PMCID: PMC10405096
- DOI: 10.1016/j.xjidi.2023.100190
Skin Infiltrate Composition as a Telling Measure of Responses to Checkpoint Inhibitors
Abstract
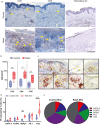
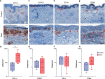
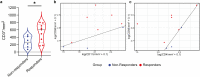
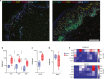
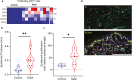
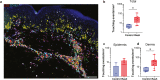
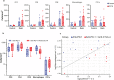
Checkpoint inhibitors treat a variety of tumor types with significant benefits. Unfortunately, these therapies come with diverse adverse events. Skin rash is observed early into treatment and might serve as an indicator of downstream responses to therapy. We studied the cellular composition of cutaneous eruptions and whether their contribution varies with the treatment applied. Skin samples from 18 patients with cancer and 11 controls were evaluated by mono- and multiplex imaging, quantification, and statistical analysis. T cells were the prime contributors to skin rash, with T cells and macrophages interacting and proliferating on site. Among T cell subsets examined, type 1 and 17 T cells were relatively increased among inflammatory skin infiltrates. A combination of increased cytotoxic T cell content and decreased macrophage abundance was associated with dual checkpoint inhibition over PD1 inhibition alone. Importantly, responders significantly separated from nonresponders by greater CD68+ macrophage and either CD11c+ antigen-presenting cell or CD4+ T cell abundance in skin rash. The microenvironment promoted epidermal proliferation and thickening as well. The combination of checkpoint inhibitors used affects the development and composition of skin infiltrates, whereas the combined abundance of two cell types in cutaneous eruptions aligns with responses to checkpoint inhibitor therapy.
© 2023 Published by Elsevier Inc. on behalf of the Society for Investigative Dermatology.
Figures

References
-
- Chistiakov D.A., Killingsworth M.C., Myasoedova V.A., Orekhov A.N., Bobryshev Y.V. CD68/macrosialin: not just a histochemical marker. Lab Invest. 2017;97:4–13. - PubMed
-
- Choi J., Anderson R., Blidner A., Cooksley T., Dougan M., Glezerman I., et al. Multinational Association of Supportive Care in Cancer (MASCC) 2020 clinical practice recommendations for the management of severe dermatological toxicities from checkpoint inhibitors. Support Care Cancer. 2020;28:6119–6128. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

