Antibacterial conductive self-healing hydrogel wound dressing with dual dynamic bonds promotes infected wound healing
- PMID: 37554541
- PMCID: PMC10404845
- DOI: 10.1016/j.bioactmat.2023.07.015
Antibacterial conductive self-healing hydrogel wound dressing with dual dynamic bonds promotes infected wound healing
Abstract
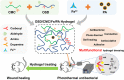
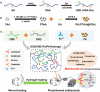
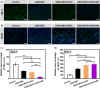
In clinical applications, there is a lack of wound dressings that combine efficient resistance to drug-resistant bacteria with good self-healing properties. In this study, a series of adhesive self-healing conductive antibacterial hydrogel dressings based on oxidized sodium alginate-grafted dopamine/carboxymethyl chitosan/Fe3+ (OSD/CMC/Fe hydrogel)/polydopamine-encapsulated poly(thiophene-3-acetic acid) (OSD/CMC/Fe/PA hydrogel) were prepared for the repair of infected wound. The Schiff base and Fe3+ coordination bonds of the hydrogel structure are dynamic bonds that can be repaired automatically after the hydrogel network is disrupted. Macroscopically, the hydrogel exhibits self-healing properties, allowing the hydrogel dressing to adapt to complex wound surfaces. The OSD/CMC/Fe/PA hydrogel showed good conductivity and photothermal antibacterial properties under near-infrared (NIR) light irradiation. In addition, the hydrogels exhibit tunable rheological properties, suitable mechanical properties, antioxidant properties, tissue adhesion properties and hemostatic properties. Furthermore, all hydrogel dressings improved wound healing in the infected full-thickness defect skin wound repair test in mice. The wound size repaired by OSD/CMC/Fe/PA3 hydrogel + NIR was much smaller (12%) than the control group treated with Tegaderm™ film after 14 days. In conclusion, the hydrogels have high antibacterial efficiency, suitable conductivity, great self-healing properties, good biocompatibility, hemostasis and antioxidant properties, making them promising candidates for wound healing dressings for the treatment of infected skin wounds.
Keywords: Dynamic crosslinking; Infected wound; Photothermal antibacterial; Self-healing; Wound healing.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Castaño O., Pérez-Amodio S., Navarro-Requena C., Mateos-Timoneda M.Á., Engel E. Instructive microenvironments in skin wound healing: biomaterials as signal releasing platforms. Adv. Drug Deliv. Rev. 2018;129:95–117. - PubMed
-
- Wang M., Luo Y., Wang T., Wan C., Pan L., Pan S., He K., Neo A., Chen X. Artificial skin perception. Adv. Mater. 2021;33(19) - PubMed
-
- Zhao X., Liang Y., Huang Y., He J., Han Y., Guo B. Physical double-network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug-resistant bacterial infection and removable wound dressing. Adv. Funct. Mater. 2020;30(17)
-
- Schoenenberger A.D., Tempfer H., Lehner C., Egloff J., Mauracher M., Bird A., Widmer J., Maniura-Weber K., Fucentese S.F., Traweger A., Silvan U., Snedeker J.G. Macromechanics and polycaprolactone fiber organization drive macrophage polarization and regulate inflammatory activation of tendon in vitro and in vivo. Biomaterials. 2020;249 - PubMed
-
- Wei X., Ding S., Liu S., Yang K., Cai J., Li F., Wang C., Lin S., Tian F. Polysaccharides-modified chitosan as improved and rapid hemostasis foam sponges. Carbohydr. Polym. 2021;264 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

