Exploring the Onset and Progression of Prostate Cancer through a Multicellular Agent-based Model
- PMID: 37554550
- PMCID: PMC10405859
- DOI: 10.1158/2767-9764.CRC-23-0097
Exploring the Onset and Progression of Prostate Cancer through a Multicellular Agent-based Model
Abstract
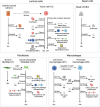
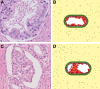
Over 10% of men will be diagnosed with prostate cancer during their lifetime. Arising from luminal cells of the prostatic acinus, prostate cancer is influenced by multiple cells in its microenvironment. To expand our knowledge and explore means to prevent and treat the disease, it is important to understand what drives the onset and early stages of prostate cancer. In this study, we developed an agent-based model of a prostatic acinus including its microenvironment, to allow for in silico studying of prostate cancer development. The model was based on prior reports and in-house data of tumor cells cocultured with cancer-associated fibroblasts (CAF) and protumor and/or antitumor macrophages. Growth patterns depicted by the model were pathologically validated on hematoxylin and eosin slide images of human prostate cancer specimens. We identified that stochasticity of interactions between macrophages and tumor cells at early stages strongly affect tumor development. In addition, we discovered that more systematic deviations in tumor development result from a combinatorial effect of the probability of acquiring mutations and the tumor-promoting abilities of CAFs and macrophages. In silico modeled tumors were then compared with 494 patients with cancer with matching characteristics, showing strong association between predicted tumor load and patients' clinical outcome. Our findings suggest that the likelihood of tumor formation depends on a combination of stochastic events and systematic characteristics. While stochasticity cannot be controlled, information on systematic effects may aid the development of prevention strategies tailored to the molecular characteristics of an individual patient.
Significance: We developed a computational model to study which factors of the tumor microenvironment drive prostate cancer development, with potential to aid the development of new prevention strategies.
© 2023 The Authors; Published by the American Association for Cancer Research.
Figures





References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
-
- Ramon J, Denis LJ. Prostate cancer. New York (NY): Springer Science & Business Media; 2007.
-
- Davidson D, Bostwick DG, Qian J, Wollan PC, Oesterling JE, Rudders RA, et al. Prostatic intraepithelial neoplasia is a risk factor for adenocarcinoma: predictive accuracy in needle biopsies. J Urol 1995;154:1295–9. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

