Impact of elexacaftor/tezacaftor/ivacaftor on lung function, nutritional status, pulmonary exacerbation frequency and sweat chloride in people with cystic fibrosis: real-world evidence from the German CF Registry
- PMID: 37554663
- PMCID: PMC10405057
- DOI: 10.1016/j.lanepe.2023.100690
Impact of elexacaftor/tezacaftor/ivacaftor on lung function, nutritional status, pulmonary exacerbation frequency and sweat chloride in people with cystic fibrosis: real-world evidence from the German CF Registry
Abstract
Background: Treatment with elexacaftor/tezacaftor/ivacaftor (ETI) improves multiple clinical outcomes in people with cystic fibrosis (pwCF) with at least one F508del allele. This study evaluated the real-world impact of ETI on lung function, nutritional status, pulmonary exacerbation frequency, and sweat chloride concentrations in a large group of pwCF.
Methods: This observational cohort study used data from the German CF Registry for pwCF who received ETI therapy and were followed up for a period of 12 months.
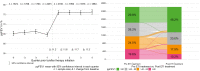
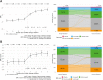
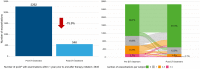
Findings: The study included 2645 pwCF from 67 centres in Germany (mean age 28.0 ± 11.5 years). Over the first year after ETI was initiated, percent predicted forced expiratory volume in 1 s (ppFEV1) increased by 11.3% (95% confidence interval [CI] 10.8-11.8, p < 0.0001), body mass index (BMI) z-score increased by 0.3 (95% CI 0.3-0.4, p < 0.0001) in individuals aged 12 to <18 years and BMI in adults increased by 1.4 kg/m2 (95% CI 1.3-1.4, p < 0.0001), pulmonary exacerbations decreased by 75.9% (p < 0.0001) and mean sweat chloride concentration decreased by 50.9 mmol/L (95% CI -52.6, -49.3, p < 0.0001). Improvements in ppFEV1 over the first year of therapy were greater in pwCF who had not previously received cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapy (12.6% [95% CI 11.9-13.4] vs. 9.7% [95% CI 9.0-10.5] in those with prior CFTR modulator treatment.
Interpretation: These real-world data are consistent with the findings of randomised clinical trials, and support the use of ETI as a highly effective treatment option for pwCF who have at least one F508del allele.
Funding: None.
Keywords: Body mass index; Cystic fibrosis; Elexacaftor/tezacaftor/ivacaftor; Lung function; Pulmonary exacerbation; Real-world evidence; Sweat chloride.
© 2023 The Author(s).
Conflict of interest statement
SS received personal fees or grants from Galapagos, Proteostasis Therapeutics, Celtaxsys, Vertex Pharmaceuticals, Boehringer Ingelheim, Corbus Pharmaceuticals, Insmed Germany GmbH and Ionis Pharmaceuticals outside the submitted work. SD participated in the Advance program, financially supported by Vertex Pharmaceuticals. MW received personal fees from Vertex Pharmaceuticals, Chiesi, CSL Behring, and Grifols outside of the submitted work. CSm received personal fees from Vertex Pharmaceuticals outside the submitted work. FS has no conflict of interest. FB has no conflict of interest. MB receives payments from Mukoviszidose Institut gGmbH. AMD received personal fees from Vertex outside of the submitted work and institutional payments from Vertex for the conduct of clinical studies. HE received personal fees Vertex Pharmaceuticals and Insmed Germany GmbH outside the submitted work. CS received personal fees or grants from Chiesi, GlaxoSmithKline, Boehringer Ingelheim, Vertex Pharmaceuticals and GILEAD outside the submitted work. OE received personal fees or grants from Boerhringer Ingelheim, Chiesi, Corbus Pharmaceuticals, GILEAD, Novartis, Vertex Pharmaceuticals outside the submitted work. MK has no conflict of interest. SaS receives payments for statistical analysis of data that were made to STAT-UP Statistical Consulting & Data Science GmbH. SN received institutional payments from Vertex for the conduct of clinical studies. LN received institutional payments from The German Center of Lung research and Vertex Pharmaceuticals for the conduct of clinical studies, was the medical lead of the German CF Registry and the Pharmacovigilance Study manager of the European Cystic Fibrosis Society Patient Registry and received Medial Writing support from Articulate Science.
Figures
References
-
- Guo J., Garratt A., Hill A. Worldwide rates of diagnosis and effective treatment for cystic fibrosis. J Cyst Fibros. 2022;21(3):456–462. - PubMed
-
- Naehrlich L., Burkhart M., Basler C., et al. Mukoviszidose e.V.; Bonn: 2021. Annual report German CF registry.
-
- Shteinberg M., Haq I.J., Polineni D., Davies J.C. Cystic fibrosis. Lancet. 2021;397(10290):2195–2211. - PubMed
LinkOut - more resources
Full Text Sources

