Self-assembled GLP-1/glucagon peptide nanofibrils prolong inhibition of food intake
- PMID: 37554763
- PMCID: PMC10406450
- DOI: 10.3389/fendo.2023.1217021
Self-assembled GLP-1/glucagon peptide nanofibrils prolong inhibition of food intake
Abstract
Introduction: Oxyntomodulin (Oxm) hormone peptide has a number of beneficial effects on nutrition and metabolism including increased energy expenditure and reduced body weight gain. Despite its many advantages as a potential therapeutic agent, Oxm is subjected to rapid renal clearance and protease degradation limiting its clinical application. Previously, we have shown that subcutaneous administration of a fibrillar Oxm formulation can significantly prolong its bioactivity in vivo from a few hours to a few days.
Methods: We used a protease resistant analogue of Oxm, Aib2-Oxm, to form nanfibrils depot and improve serum stability of released peptide. The nanofibrils and monomeric peptide in solution were characterized by spectroscopic, microscopic techniques, potency assay, QCM-D and in vivo studies.
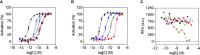
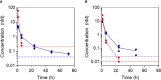
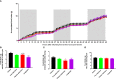
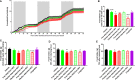
Results: We show that in comparison to Oxm, Aib2-Oxm fibrils display a slower elongation rate requiring higher ionic strength solutions, and a higher propensity to dissociate. Upon subcutaneous administration of fibrillar Aib2-Oxm in rodents, a 5-fold increase in bioactivity relative to fibrillar Oxm and a significantly longer bioactivity than free Aib2-Oxm were characterized. Importantly, a decrease in food intake was observed up to 72-hour post-administration, which was not seen for free Aib2-Oxm.
Conclusion: Our findings provides compelling evidence for the development of long-lasting peptide fibrillar formulations that yield extended plasma exposure and enhanced in vivo pharmacological response.
Keywords: GLP-1/glucagon; depot formulations; metabolic diseases; nanofibrils; peptides; self-assembly.
Copyright © 2023 Ouberai, Gomes Dos Santos, Kinna, Hornigold, Baker, Naylor, Liang, Corkill and Welland.
Conflict of interest statement
Authors AG, DH, DB, JN, LL and DC were employed by AstraZeneca. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

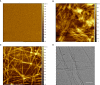

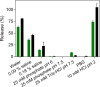
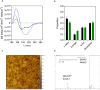
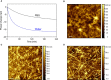
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

