Identification and validation of a novel anoikis-related signature for predicting prognosis and immune landscape in ovarian serous cystadenocarcinoma
- PMID: 37554782
- PMCID: PMC10404752
- DOI: 10.1016/j.heliyon.2023.e18708
Identification and validation of a novel anoikis-related signature for predicting prognosis and immune landscape in ovarian serous cystadenocarcinoma
Abstract
Background: Ovarian serous cystadenocarcinoma (OSC) is the most prevalent histological subtype of ovarian cancer (OV) and presents a serious threat to women's health. Anoikis is an essential component of metastasis, and tumor cells can get beyond it to become viable. The impact of anoikis on OSC, however, has only been the topic of a few studies.
Methods: The mRNA sequencing and clinical information of OSC came from The Cancer Genome Atlas Target Genotype-Tissue Expression (TCGA TARGET GTEx) dataset. Anoikis-related genes (ARGs) were collected by Harmonizome and GeneCards websites. Centered on these ARGs, we used unsupervised consensus clustering to explore potential tumor typing and filtered hub ARGs to create a model of predictive signature for OSC patients. Furthermore, we presented clinical specialists with a novel nomogram based on ARGs, revealing the underlying clinical relevance of this signature. Finally, we explored the immune microenvironment among various risk groups.
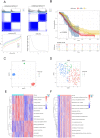
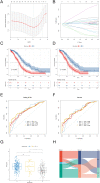
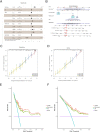
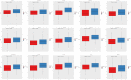
Results: We identified 24 ARGs associated with the prognosis of OSC and classified OSC patients into three subtypes, and the subtype with the best prognosis was more enriched in immune-related pathways. Seven ARGs (ARHGEF7, NOTCH4, CASP2, SKP2, PAK4, LCK, CCDC80) were chosen to establish a risk model and a nomogram that can provide practical clinical decision support. Risk scores were found to be an independent and significant prognostic factor in OSC patients. The CIBERSORTx result revealed an inflammatory microenvironment is different for risk groups, and the proportion of immune infiltrates of Macrophages M1 is negatively correlated with risk score (rs = -0.21, P < 0.05). Ultimately, quantitative reverse transcription polymerase chain reaction (RT-PCR) was utilized to validate the expression of the seven pivotal ARGs.
Conclusion: In this study, based on seven ARGs, a risk model and nomogram established can be used for risk stratification and prediction of survival outcomes in patients with OSC, providing a reliable reference for individualized therapy of OSC patients.
Keywords: Anoikis; Immune landscape; Ovarian serous cystadenocarcinoma; Prognosis; Tumor metastasis.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






Similar articles
-
Identification of a novel anoikis-related gene signature to predict prognosis and tumor microenvironment in lung adenocarcinoma.Thorac Cancer. 2023 Jan;14(3):320-330. doi: 10.1111/1759-7714.14766. Epub 2022 Dec 11. Thorac Cancer. 2023. PMID: 36507553 Free PMC article.
-
Establishment and validation of a novel anoikis-related prognostic signature of clear cell renal cell carcinoma.Front Immunol. 2023 Mar 28;14:1171883. doi: 10.3389/fimmu.2023.1171883. eCollection 2023. Front Immunol. 2023. PMID: 37056778 Free PMC article.
-
Anoikis-related genes in breast cancer patients: reliable biomarker of prognosis.BMC Cancer. 2024 Sep 19;24(1):1163. doi: 10.1186/s12885-024-12830-5. BMC Cancer. 2024. PMID: 39300389 Free PMC article.
-
Prognosis prediction and drug guidance of ovarian serous cystadenocarcinoma through mitochondria gene-based model.Cancer Genet. 2025 Apr;292-293:1-13. doi: 10.1016/j.cancergen.2024.12.005. Epub 2024 Dec 29. Cancer Genet. 2025. PMID: 39754905
-
A novel anoikis-related prognostic signature associated with prognosis and immune infiltration landscape in clear cell renal cell carcinoma.Front Genet. 2022 Oct 19;13:1039465. doi: 10.3389/fgene.2022.1039465. eCollection 2022. Front Genet. 2022. PMID: 36338978 Free PMC article.
Cited by
-
Identification and single-cell analysis of prognostic genes related to mitochondrial and neutrophil extracellular traps in bladder cancer.Sci Rep. 2025 Jul 4;15(1):23982. doi: 10.1038/s41598-025-10413-3. Sci Rep. 2025. PMID: 40615671 Free PMC article.
-
Anoikis in cell fate, physiopathology, and therapeutic interventions.MedComm (2020). 2024 Sep 15;5(10):e718. doi: 10.1002/mco2.718. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39286778 Free PMC article. Review.
References
-
- Matulonis U.A. Management of newly diagnosed or recurrent ovarian cancer. Clin. Adv. Hematol. Oncol. 2018;16(6):426–437. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

