Enhancing interventions for prevention of mother-to-child- transmission of hepatitis B virus
- PMID: 37554925
- PMCID: PMC10405098
- DOI: 10.1016/j.jhepr.2023.100777
Enhancing interventions for prevention of mother-to-child- transmission of hepatitis B virus
Abstract
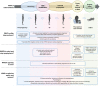
Prevention of mother-to-child transmission of hepatitis B virus (HBV) infection is a cornerstone of efforts to support progress towards elimination of viral hepatitis. Current guidelines recommend maternal screening, antiviral therapy during the third trimester of high-risk pregnancies, universal and timely HBV birth dose vaccination, and post-exposure prophylaxis with hepatitis B immunoglobulin for selected neonates. However, serological and molecular diagnostic testing, treatment and HBV vaccination are not consistently deployed, particularly in many high endemicity settings, and models predict that global targets for reduction in paediatric incidence will not be met by 2030. In this article, we briefly summarise the evidence for current practice and use this as a basis to discuss areas in which prevention of mother-to-child transmission can potentially be enhanced. By reducing health inequities, enhancing pragmatic use of resources, filling data gaps, developing advocacy and education, and seeking consistent investment from multilateral agencies, significant advances can be made to further reduce vertical transmission events, with wide health, societal and economic benefits.
Keywords: HBIG; HBV; PMTCT; birth dose; elimination; hepatitis B virus; prevention; tenofovir; transmission; vaccination; vertical transmission.
© 2023 The Author(s).
Conflict of interest statement
CWS has received speaker fees from GILEAD Sciences and Abbott. CP received research funding and is a speaker for Gilead. PCM supervises a doctoral student with funding support from GSK. SH has received funding from Gilead for HCV Micro-elimination programs. HR has received research funding and speaker’s honoraria from Gilead Sciences, AbbVie and Pfizer and is a board member of the CDA foundation. SW has received research funding from Gilead Sciences, and honoraria from Prime Inc, and is on the Board of Directors for the Hepatitis B Foundation, World Hepatitis Alliance, and serves in the patient advisory group and HBV special interest group for the AASLD. FR has received consulting fees from Sanofi Pasteur. GD has received consulting fees and speaker fees from Gilead Sciences, has participated on Data Safety Monitoring Board/Advisory Board for janssen, Glaxo Smith Kline, Arbutus, Aligos, Vir and has roles in the National Medical Research Council Singapore and the World Health Organisation Pediatric Working Group on Viral Hepatitis. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures
References
-
- World Health Organization . 2016. Towards Ending Viral Hepat. Global health sector strategy on viral hepatitis 2016–2021.https://apps.who.int/iris/handle/10665/246177 WHO/HIV/2016.06.
-
- WHO . June 2022. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030.Https://cdn.who.int/media/docs/default-Source/hq-Hiv-Hepatitis-and-Stis-... Published on-line. [n.d]
-
- Edmunds W.J., Medley G.F., Nokes D.J., Hall A.J., Whittle H.C. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253:197–201. - PubMed
-
- Nayagam S., Thursz M., Sicuri E., Conteh L., Wiktor S., Low-Beer D., et al. Requirements for global elimination of hepatitis B: a modelling study. Lancet Infect Dis. 2016;16:1399–1408. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

