Decoding the Brain's Surface to Track Deeper Activity
- PMID: 37555135
- PMCID: PMC10406232
- DOI: 10.3389/fnimg.2022.815778
Decoding the Brain's Surface to Track Deeper Activity
Abstract
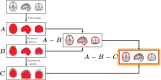
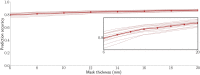
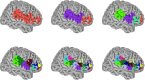
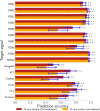
Neural activity can be readily and non-invasively recorded from the scalp using electromagnetic and optical signals, but unfortunately all scalp-based techniques have depth-dependent sensitivities. We hypothesize, though, that the cortex's connectivity with the rest of the brain could serve to construct proxy signals of deeper brain activity. For example, functional magnetic resonance imaging (fMRI)-derived models that link surface connectivity to deeper regions could subsequently extend the depth capabilities of other modalities. Thus, as a first step toward this goal, this study examines whether or not surface-limited support vector regression of resting-state fMRI can indeed track deeper regions and distributed networks in independent data. Our results demonstrate that depth-limited fMRI signals can in fact be calibrated to report ongoing activity of deeper brain structures. Although much future work remains to be done, the present study suggests that scalp recordings have the potential to ultimately overcome their intrinsic physical limitations by utilizing the multivariate information exchanged between the surface and the rest of the brain.
Keywords: cerebral cortex; functional magnetic resonance imaging; multimodal; resting state connectivity; support vector machine.
Copyright © 2022 Tenzer, Lisinski and LaConte.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources

