The combination of atomoxetine and oxybutynin for the treatment of obstructive sleep apnea in children with Down syndrome
- PMID: 37555595
- PMCID: PMC10692944
- DOI: 10.5664/jcsm.10764
The combination of atomoxetine and oxybutynin for the treatment of obstructive sleep apnea in children with Down syndrome
Abstract
Study objectives: Children with Down syndrome (DS) are at very high risk for obstructive sleep apnea (OSA). Current OSA treatments have limited effectiveness in this population. We evaluated the effectiveness of atomoxetine and oxybutynin (ato-oxy) to treat OSA in children with Down syndrome.
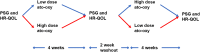
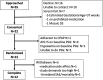
Methods: Children ages 6-7 years old with Down syndrome and OSA participated in a double-blind crossover clinical trial evaluating two dose regimens of ato-oxy. Participants received low-dose ato-oxy (0.5 mg/kg atomoxetine and 5 mg oxybutynin) and high-dose ato-oxy (1.2 mg/kg atomoxetine and 5 mg oxybutynin) for 1 month in random order. The primary study outcome was change in obstructive apnea-hypopnea index. Health-related quality of life as measured by the OSA-18 as well as changes in sleep architecture were secondary outcomes.
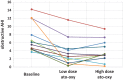
Results: Fifteen participants qualified for randomization and 11 participants had complete data at all points. Baseline obstructive apnea-hypopnea index was 7.4 ± 3.7 (mean ± standard deviation), obstructive apnea-hypopnea index with low-dose ato-oxy was 3.6 ± 3.3 (P = .001 vs baseline), and obstructive apnea-hypopnea index with high-dose ato-oxy was 3.9 ± 2.8 (P = .003 vs baseline). No significant sleep architecture differences were present with ato-oxy. No significant difference in OSA-18 score was present. OSA-18 total score was 51 ± 19 at baseline, 45 ± 17 (P = .09) at the end of 4 weeks of low-dose ato-oxy, and 45 ± 16 (P = .37) at the end of high-dose ato-oxy therapy. The most common adverse effects were irritability and fatigue, and these were generally mild.
Conclusions: Ato-oxy is a promising treatment for OSA in children with Down syndrome.
Clinical trial registration: Registry: Clinicaltrials.gov; Name: Medications for Obstructive Sleep Apnea In Children With Down Syndrome (MOSAIC); URL: https://clinicaltrials.gov/ct2/show/NCT04115878; Identifier: NCT04115878.
Citation: Combs D, Edgin J, Hsu C-H, et al. The combination of atomoxetine and oxybutynin for the treatment of obstructive sleep apnea in children with Down syndrome. J Clin Sleep Med. 2023;19(12):2065-2073.
Keywords: Down syndrome; obstructive sleep apnea.
© 2023 American Academy of Sleep Medicine.
Conflict of interest statement
All authors have seen and approved the manuscript. Funding provided by the National Institutes of Health Include (INvestigation of Co-occurring conditions across the Lifespan to Understand Down syndrome) project: HL151254 and HD109777, as well as the Lumind-IDSC foundation. D.C. reports a grant from the American Heart Association. J.E. reports grants from the Lejeune Foundation, the LuMind Foundation, and the National Institutes of Health. S.P. reports grants from the National Institutes of Health, American Academy of Sleep Medicine Foundation, Patient Centered Outcomes Research Institute, personal royalty fees from UpToDate Inc, and consultant fees from Jazz pharmaceuticals. Outside the submitted work, S.P. has a patent US20160213879A1 (home breathing device) that is licensed to SaiOx, Inc. C.-H.H., K.B., H.V.V., S.L.R., B.G., and D.M. have no relationships to disclose.
Figures
References
-
- Parker SE , Mai CT , Canfield MA , et al. National Birth Defects Prevention Network . Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004-2006 . Birth Defects Res A Clin Mol Teratol. 2010. ; 88 ( 12 ): 1008 – 1016 . - PubMed
-
- Marcus CL , Brooks LJ , Draper KA , et al. American Academy of Pediatrics . Diagnosis and management of childhood obstructive sleep apnea syndrome . Pediatrics. 2012. ; 130 ( 3 ): 576 – 584 . - PubMed
-
- Shott SR , Amin R , Chini B , Heubi C , Hotze S , Akers R . Obstructive sleep apnea: should all children with Down syndrome be tested? Arch Otolaryngol Head Neck Surg. 2006. ; 132 ( 4 ): 432 – 436 . - PubMed
-
- Hill CM , Evans HJ , Elphick H , et al . Prevalence and predictors of obstructive sleep apnoea in young children with Down syndrome . Sleep Med. 2016. ; 27-28 : 99 – 106 . - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

