Ubiquitin-targeted bacterial effectors: rule breakers of the ubiquitin system
- PMID: 37555693
- PMCID: PMC10505922
- DOI: 10.15252/embj.2023114318
Ubiquitin-targeted bacterial effectors: rule breakers of the ubiquitin system
Abstract
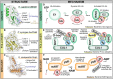
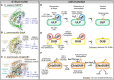
Regulation through post-translational ubiquitin signaling underlies a large portion of eukaryotic biology. This has not gone unnoticed by invading pathogens, many of which have evolved mechanisms to manipulate or subvert the host ubiquitin system. Bacteria are particularly adept at this and rely heavily upon ubiquitin-targeted virulence factors for invasion and replication. Despite lacking a conventional ubiquitin system of their own, many bacterial ubiquitin regulators loosely follow the structural and mechanistic rules established by eukaryotic ubiquitin machinery. Others completely break these rules and have evolved novel structural folds, exhibit distinct mechanisms of regulation, or catalyze foreign ubiquitin modifications. Studying these interactions can not only reveal important aspects of bacterial pathogenesis but also shed light on unexplored areas of ubiquitin signaling and regulation. In this review, we discuss the methods by which bacteria manipulate host ubiquitin and highlight aspects that follow or break the rules of ubiquitination.
Keywords: Ubiquitin; bacterial effector; bacterial pathogenesis; post-translational modification.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Abramovitch RB, Martin GB (2005) AvrPtoB: a bacterial type III effector that both elicits and suppresses programmed cell death associated with plant immunity. FEMS Microbiol Lett 245: 1–8 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

