Topical Drug Delivery of Concentrated Cabazitaxel in an α-Tocopherol and DMSO Solution
- PMID: 37555802
- PMCID: PMC10582425
- DOI: 10.1002/advs.202302658
Topical Drug Delivery of Concentrated Cabazitaxel in an α-Tocopherol and DMSO Solution
Abstract
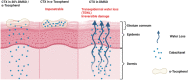
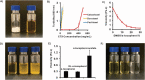
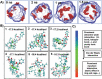
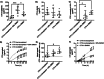
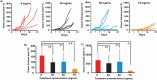
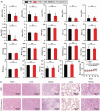
Topical chemotherapy approaches are relevant for certain skin cancer treatments. This study observes that cabazitaxel (CTX), a broad-spectrum second-generation taxane cytotoxic agent, can be dissolved in α-tocopherol at high concentrations exceeding 100 mg mL-1 . 2D nuclear magnetic resonance (NMR) analysis and molecular dynamics (MD) are used to study this phenomenon. The addition of 30% dimethyl sulfoxide (DMSO) to the α-tocopherol/CTX solution improves its working viscosity and enhances CTX permeation through human skin in vitro (over 5 µg cm-2 within 24 h), while no detectable drug permeates when CTX is dissolved in α-tocopherol alone. In a transepidermal water loss assay, the barrier impairment induced by CTX in 30% DMSO in α-tocopherol, but not in pure DMSO, is reversible 8 h after the formulation removal from the skin surface. Antitumor efficacy of the topical CTX formulation is evaluated in nude mice bearing A431 human squamous carcinoma skin cancer xenografts. With topical application of concentrated CTX solutions (75 mg mL-1 ), tumor growth is significantly suppressed compared to lower concentration groups (0, 25, or 50 mg mL-1 CTX). Taken together, these findings show that topical delivery of CTX using a DMSO and α-tocopherol solvent warrants further study as a treatment for skin malignancies.
Keywords: cabazitaxel; skin cancer; transdermal delivery; α-tocopherol.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Rembielak A., Ajithkumar T., Clin. Oncol. 2019, 31, 735. - PubMed
-
- Ciążyńska M., Kamińska‐Winciorek G., Lange D., Lewandowski B., Reich A., Sławińska M., Pabianek M., Szczepaniak K., Hankiewicz A., Ułańska M., Morawiec J., Błasińska‐Morawiec M., Morawiec Z., Piekarski J., Nejc D., Brodowski R., Zaryczańska A., Sobjanek M., Nowicki R. J., Owczarek W., Słowińska M., Wróbel K., Bieniek A., Woźniacka A., Skibińska M., Narbutt J., Niemczyk W., Ciążyński K., Lesiak A., Sci. Rep. 2021, 11, 4337. - PMC - PubMed
-
- Simões M. C. F., Sousa J. J. S., Pais A., Cancer Lett. 2015, 357, 8. - PubMed
-
- Khan M. A., Pandit J., Sultana Y., Sultana S., Ali A., Aqil M., Chauhan M., Drug Deliv. 2015, 22, 795. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
