Self-Assembled Nanostructures in Aprotic Ionic Liquids Facilitate Charge Transport at Elevated Pressure
- PMID: 37555825
- PMCID: PMC10450691
- DOI: 10.1021/acsami.3c08606
Self-Assembled Nanostructures in Aprotic Ionic Liquids Facilitate Charge Transport at Elevated Pressure
Abstract
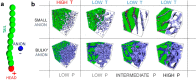
Ionic liquids (ILs), revealing a tendency to form self-assembled nanostructures, have emerged as promising materials in various applications, especially in energy storage and conversion. Despite multiple reports discussing the effect of structural factors and external thermodynamic variables on ion organization in a liquid state, little is known about the charge-transport mechanism through the self-assembled nanostructures and how it changes at elevated pressure. To address these issues, we chose three amphiphilic ionic liquids containing the same tetra(alkyl)phosphonium cation and anions differing in size and shape, i.e., thiocyanate [SCN]-, dicyanamide [DCA]-, and tricyanomethanide [TCM]-. From ambient pressure dielectric and mechanical experiments, we found that charge transport of all three examined ILs is viscosity-controlled at high temperatures. On the other hand, ion diffusion is much faster than structural dynamics in a nanostructured supercooled liquid (at T < 210 ± 3 K), which constitutes the first example of conductivity independent from viscosity in neat aprotic ILs. High-pressure measurements and MD simulations reveal that the created nanostructures depend on the anion size and can be modified by compression. For small anions, increasing pressure shapes immobile alkyl chains into lamellar-type phases, leading to increased anisotropic diffusivity of anions through channels. Bulky anions drive the formation of interconnected phases with continuous 3D curvature, which render ion transport independent of pressure. This work offers insight into the design of high-density electrolytes with percolating conductive phases providing efficient ion flow.
Keywords: charge transport mechanism; high pressure; ionic liquids; liquid−liquid phase transition; self-assembly.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
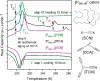


 (right axis). Avrami–Avramov plot
constructed for [P666,14][DCA] at 209 K (left axis). (d)
Rate constant k of the crystallization process as
a function of inverse temperature.
(right axis). Avrami–Avramov plot
constructed for [P666,14][DCA] at 209 K (left axis). (d)
Rate constant k of the crystallization process as
a function of inverse temperature.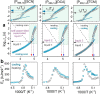
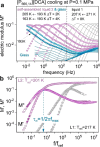
 to experimental data. Dashed lines indicate Tg and the temperature of LLT (blue and red arrows).
Zooms highlight different τσ(Tg) obtained on cooling (blue arrow) and heating (gray
arrow) scans. (b) Apparent activation energy Ea =
to experimental data. Dashed lines indicate Tg and the temperature of LLT (blue and red arrows).
Zooms highlight different τσ(Tg) obtained on cooling (blue arrow) and heating (gray
arrow) scans. (b) Apparent activation energy Ea =  calculated from dielectric data
obtained
on cooling and heating for [P666,14][SCN], [P666,14][DCA], and [P666,14][TCM].
calculated from dielectric data
obtained
on cooling and heating for [P666,14][SCN], [P666,14][DCA], and [P666,14][TCM].

References
-
- MacFarlane D. R.; Forsyth M.; Howlett P. C.; Kar M.; Passerini S.; Pringle J. M.; Ohno H.; Watanabe M.; Yan F.; Zheng W.; Zhang S.; Zhang J. Ionic Liquids and Their Solid-State Analogues as Materials for Energy Generation and Storage. Nat. Rev. Mater. 2016, 1, 15005. 10.1038/natrevmats.2015.5. - DOI
-
- Wojnarowska Z.; Grzybowska K.; Hawelek L.; Swiety-Pospiech A.; Masiewicz E.; Paluch M.; Sawicki W.; Chmielewska A.; Bujak P.; Markowski J. Molecular Dynamics Studies on the Water Mixtures of Pharmaceutically Important Ionic Liquid Lidocaine HCl. Mol. Pharmaceutics 2012, 9 (5), 1250–1261. 10.1021/mp2005609. - DOI - PubMed
LinkOut - more resources
Full Text Sources

