Regulating NLRP3 Inflammasome-Induced Pyroptosis via Nrf2: TBHQ Limits Hyperoxia-Induced Lung Injury in a Mouse Model of Bronchopulmonary Dysplasia
- PMID: 37556072
- PMCID: PMC10673969
- DOI: 10.1007/s10753-023-01885-4
Regulating NLRP3 Inflammasome-Induced Pyroptosis via Nrf2: TBHQ Limits Hyperoxia-Induced Lung Injury in a Mouse Model of Bronchopulmonary Dysplasia
Abstract
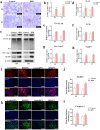
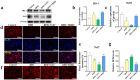
Nuclear factor e2-related factor 2 (Nrf2) plays a key role in cellular resistance to oxidative stress injury. Oxidative stress injury, caused by Nrf2 imbalance, results in increased pyroptosis, DNA damage, and inflammatory activation, which may lead to the arrest of alveolar development and bronchopulmonary dysplasia (BPD) in premature infants under hyperoxic conditions. We established a BPD mouse model to investigate the effects of tert-butylhydroquinone (TBHQ), an Nrf2 activator, on oxidative stress injury, pyroptosis, NLRP3 inflammasome activation, and alveolar development. TBHQ reduced abnormal cell death in the lung tissue of BPD mice and restored the number and normal structure of the alveoli. TBHQ administration activated the Nrf2/heme oxygenase-1 (HO-1) signaling pathway, resulting in the decrease in the following: reactive oxygen species (ROS), activation of the NOD-like receptor pyrin domain containing 3 (NLRP3) inflammasome, and IL-18 and IL-1β expression and activation, as well as inhibition of pyroptosis. In contrast, after Nrf2 gene knockout in BPD mice, there was more severe oxidative stress injury and cell death in the lungs, there were TUNEL + and NLRP3 + co-positive cells in the alveoli, the pyroptosis was significantly increased, and the development of alveoli was significantly blocked. We demonstrated that TBHQ may promote alveolar development by enhancing Nrf2-induced antioxidation in the lung tissue of BPD mice and that the decrease in the NLRP3 inflammasome and pyroptosis caused by Nrf2 activation may be the underlying mechanism. These results suggest that TBHQ is a promising treatment for lung injury in premature infants with hyperoxia.
Keywords: NLRP3; Nrf2; ROS; TBHQ; bronchopulmonary dysplasia; pyroptosis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Bhattacharya, S., J.A. Mereness, A.M. Baran, R.S. Misra, D.R. Peterson, R.M. Ryan, et al. 2021. Lymphocyte-specific biomarkers associated with preterm birth and bronchopulmonary dysplasia. Frontiers in Immunology 11: 563473. 10.3389/fimmu.2020.563473. (PubMed: 33552042, PubMedCentral: PMC7859626). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

