The genome-scale metabolic model for the purple non-sulfur bacterium Rhodopseudomonas palustris Bis A53 accurately predicts phenotypes under chemoheterotrophic, chemoautotrophic, photoheterotrophic, and photoautotrophic growth conditions
- PMID: 37556472
- PMCID: PMC10441798
- DOI: 10.1371/journal.pcbi.1011371
The genome-scale metabolic model for the purple non-sulfur bacterium Rhodopseudomonas palustris Bis A53 accurately predicts phenotypes under chemoheterotrophic, chemoautotrophic, photoheterotrophic, and photoautotrophic growth conditions
Abstract
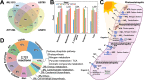
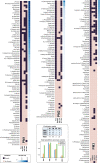
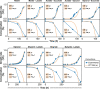
The purple non-sulfur bacterium Rhodopseudomonas palustris is recognized as a critical microorganism in the nitrogen and carbon cycle and one of the most common members in wastewater treatment communities. This bacterium is metabolically extremely versatile. It is capable of heterotrophic growth under aerobic and anaerobic conditions, but also able to grow photoautotrophically as well as mixotrophically. Therefore R. palustris can adapt to multiple environments and establish commensal relationships with other organisms, expressing various enzymes supporting degradation of amino acids, carbohydrates, nucleotides, and complex polymers. Moreover, R. palustris can degrade a wide range of pollutants under anaerobic conditions, e.g., aromatic compounds such as benzoate and caffeate, enabling it to thrive in chemically contaminated environments. However, many metabolic mechanisms employed by R. palustris to breakdown and assimilate different carbon and nitrogen sources under chemoheterotrophic or photoheterotrophic conditions remain unknown. Systems biology approaches, such as metabolic modeling, have been employed extensively to unravel complex mechanisms of metabolism. Previously, metabolic models have been reconstructed to study selected capabilities of R. palustris under limited experimental conditions. Here, we developed a comprehensive metabolic model (M-model) for R. palustris Bis A53 (iDT1294) consisting of 2,721 reactions, 2,123 metabolites, and comprising 1,294 genes. We validated the model using high-throughput phenotypic, physiological, and kinetic data, testing over 350 growth conditions. iDT1294 achieved a prediction accuracy of 90% for growth with various carbon and nitrogen sources and close to 80% for assimilation of aromatic compounds. Moreover, the M-model accurately predicts dynamic changes of growth and substrate consumption rates over time under nine chemoheterotrophic conditions and demonstrated high precision in predicting metabolic changes between photoheterotrophic and photoautotrophic conditions. This comprehensive M-model will help to elucidate metabolic processes associated with the assimilation of multiple carbon and nitrogen sources, anoxygenic photosynthesis, aromatic compound degradation, as well as production of molecular hydrogen and polyhydroxybutyrate.
Copyright: © 2023 Tec-Campos et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Oh Y. Photoproduction of hydrogen from acetate by a chemoheterotrophic bacterium Rhodopseudomonas palustris P4. Int J Hydrogen Energy. 2004. doi: 10.1016/j.ijhydene.2003.11.008 - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Miscellaneous

