Core mitochondrial genes are down-regulated during SARS-CoV-2 infection of rodent and human hosts
- PMID: 37556555
- PMCID: PMC11624572
- DOI: 10.1126/scitranslmed.abq1533
Core mitochondrial genes are down-regulated during SARS-CoV-2 infection of rodent and human hosts
Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral proteins bind to host mitochondrial proteins, likely inhibiting oxidative phosphorylation (OXPHOS) and stimulating glycolysis. We analyzed mitochondrial gene expression in nasopharyngeal and autopsy tissues from patients with coronavirus disease 2019 (COVID-19). In nasopharyngeal samples with declining viral titers, the virus blocked the transcription of a subset of nuclear DNA (nDNA)-encoded mitochondrial OXPHOS genes, induced the expression of microRNA 2392, activated HIF-1α to induce glycolysis, and activated host immune defenses including the integrated stress response. In autopsy tissues from patients with COVID-19, SARS-CoV-2 was no longer present, and mitochondrial gene transcription had recovered in the lungs. However, nDNA mitochondrial gene expression remained suppressed in autopsy tissue from the heart and, to a lesser extent, kidney, and liver, whereas mitochondrial DNA transcription was induced and host-immune defense pathways were activated. During early SARS-CoV-2 infection of hamsters with peak lung viral load, mitochondrial gene expression in the lung was minimally perturbed but was down-regulated in the cerebellum and up-regulated in the striatum even though no SARS-CoV-2 was detected in the brain. During the mid-phase SARS-CoV-2 infection of mice, mitochondrial gene expression was starting to recover in mouse lungs. These data suggest that when the viral titer first peaks, there is a systemic host response followed by viral suppression of mitochondrial gene transcription and induction of glycolysis leading to the deployment of antiviral immune defenses. Even when the virus was cleared and lung mitochondrial function had recovered, mitochondrial function in the heart, kidney, liver, and lymph nodes remained impaired, potentially leading to severe COVID-19 pathology.
Conflict of interest statement
Figures

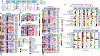





References
-
- Ramachandran K, Maity S, Muthukumar AR, Kandala S, Tomar D, Abd el-Aziz TM, Allen C, Sun Y, Venkatesan M, Madaris TR, Chiem K, Truitt R, Vishnu N, Aune G, Anderson A, Martinez-Sobrido L, Yang W, Stockand JD, Singh BB, Srikantan S, Reeves WB, Madesh M, SARS-CoV-2 infection enhances mitochondrial PTP complex activity to perturb cardiac energetics. iScience 25, 103722 (2022). - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

