A gut-restricted glutamate carboxypeptidase II inhibitor reduces monocytic inflammation and improves preclinical colitis
- PMID: 37556558
- PMCID: PMC10661206
- DOI: 10.1126/scitranslmed.abn7491
A gut-restricted glutamate carboxypeptidase II inhibitor reduces monocytic inflammation and improves preclinical colitis
Abstract
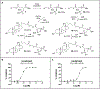
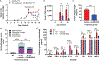
There is an urgent need to develop therapeutics for inflammatory bowel disease (IBD) because up to 40% of patients with moderate-to-severe IBD are not adequately controlled with existing drugs. Glutamate carboxypeptidase II (GCPII) has emerged as a promising therapeutic target. This enzyme is minimally expressed in normal ileum and colon, but it is markedly up-regulated in biopsies from patients with IBD and preclinical colitis models. Here, we generated a class of GCPII inhibitors designed to be gut-restricted for oral administration, and we interrogated efficacy and mechanism using in vitro and in vivo models. The lead inhibitor, (S)-IBD3540, was potent (half maximal inhibitory concentration = 4 nanomolar), selective, gut-restricted (AUCcolon/plasma > 50 in mice with colitis), and efficacious in acute and chronic rodent colitis models. In dextran sulfate sodium-induced colitis, oral (S)-IBD3540 inhibited >75% of colon GCPII activity, dose-dependently improved gross and histologic disease, and markedly attenuated monocytic inflammation. In spontaneous colitis in interleukin-10 (IL-10) knockout mice, once-daily oral (S)-IBD3540 initiated after disease onset improved disease, normalized colon histology, and attenuated inflammation as evidenced by reduced fecal lipocalin 2 and colon pro-inflammatory cytokines/chemokines, including tumor necrosis factor-α and IL-17. Using primary human colon epithelial air-liquid interface monolayers to interrogate the mechanism, we further found that (S)-IBD3540 protected against submersion-induced oxidative stress injury by decreasing barrier permeability, normalizing tight junction protein expression, and reducing procaspase-3 activation. Together, this work demonstrated that local inhibition of dysregulated gastrointestinal GCPII using the gut-restricted, orally active, small-molecule (S)-IBD3540 is a promising approach for IBD treatment.
Conflict of interest statement
Figures


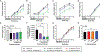
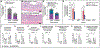


References
-
- Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB, Prevalence of inflammatory bowel disease among Adults aged ≥18 years—United States, 2015. MMWR Morb. Mortal. Wkly Rep 65, 1166–1169 (2016). - PubMed
-
- Danese S, Allez M, van Bodegraven AA, Dotan I, Gisbert JP, Hart A, Lakatos PL, Magro F, Peyrin-Biroulet L, Schreiber S, Tarabar D, Vavricka S, Halfvarson J, Vermeire S, Unmet medical needs in ulcerative colitis: An expert group consensus. Dig. Dis 37, 266–283 (2019). - PubMed
-
- Denson LA, Curran M, McGovern DPB, Koltun WA, Duerr RH, Kim SC, Sartor RB, Sylvester FA, Abraham C, de Zoeten EF, Siegel CA, Burns RM, Dobes AM, Shtraizent N, Honig G, Heller CA, Hurtado-Lorenzo A, Cho JH, Challenges in IBD research: Precision medicine. Inflamm. Bowel Dis 25, S31–S39 (2019). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

