Treating Primary Aldosteronism-Induced Hypertension: Novel Approaches and Future Outlooks
- PMID: 37556722
- PMCID: PMC10765166
- DOI: 10.1210/endrev/bnad026
Treating Primary Aldosteronism-Induced Hypertension: Novel Approaches and Future Outlooks
Abstract
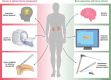
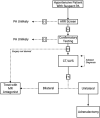
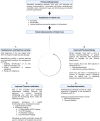
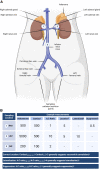
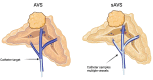
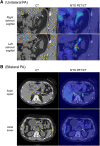
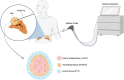
Primary aldosteronism (PA) is the most common cause of secondary hypertension and is associated with increased morbidity and mortality when compared with blood pressure-matched cases of primary hypertension. Current limitations in patient care stem from delayed recognition of the condition, limited access to key diagnostic procedures, and lack of a definitive therapy option for nonsurgical candidates. However, several recent advances have the potential to address these barriers to optimal care. From a diagnostic perspective, machine-learning algorithms have shown promise in the prediction of PA subtypes, while the development of noninvasive alternatives to adrenal vein sampling (including molecular positron emission tomography imaging) has made accurate localization of functioning adrenal nodules possible. In parallel, more selective approaches to targeting the causative aldosterone-producing adrenal adenoma/nodule (APA/APN) have emerged with the advent of partial adrenalectomy or precision ablation. Additionally, the development of novel pharmacological agents may help to mitigate off-target effects of aldosterone and improve clinical efficacy and outcomes. Here, we consider how each of these innovations might change our approach to the patient with PA, to allow more tailored investigation and treatment plans, with corresponding improvement in clinical outcomes and resource utilization, for this highly prevalent disorder.
Keywords: ablation; adrenal sparing surgery; adrenal vein sampling; functional/molecular imaging; machine learning; metabolomics; partial adrenalectomy.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Forouzanfar MH, Alexander L, Anderson HR, et al. GBD 2013 Risk factors collaborators global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990-2013: a systematic analysis for the global burden of disease study 2013. Lancet. 2015;386(10010):2287‐2323. - PMC - PubMed
-
- Sukor N. Endocrine hypertension—current understanding and comprehensive management review. Eur J Intern Med. 2011;22(5):433‐440. - PubMed
-
- Carretero OA, Oparil S. Essential hypertension: part I: definition and etiology. Circulation. 2000;101(3):329‐335. - PubMed
-
- Käyser SC, Dekkers T, Groenewoud HJ, et al. Study heterogeneity and estimation of prevalence of primary aldosteronism: a systematic review and meta-regression analysis. J Clin Endocrinol Metab. 2016;101(7):2826‐2835. - PubMed
-
- Buffolo F, Monticone S, Tetti M, Mulatero P. Primary aldosteronism in the primary care setting. Curr Opin Endocrinol Diabetes Obes. 2018;25(3):155‐159. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

