Remdesivir Reduced Mortality in Immunocompromised Patients Hospitalized for COVID-19 Across Variant Waves: Findings From Routine Clinical Practice
- PMID: 37556727
- PMCID: PMC10724457
- DOI: 10.1093/cid/ciad460
Remdesivir Reduced Mortality in Immunocompromised Patients Hospitalized for COVID-19 Across Variant Waves: Findings From Routine Clinical Practice
Abstract
Background: Immunocompromised patients are at high risk of severe coronavirus disease 2019 (COVID-19) and death, yet treatment strategies for immunocompromised patients hospitalized for COVID-19 reflect variations in clinical practice. In this comparative effectiveness study, we investigated the effect of remdesivir treatment on inpatient mortality among immunocompromised patients hospitalized for COVID-19 across all variants of concern (VOC) periods.
Methods: Data for immunocompromised patients hospitalized for COVID-19 between December 2020 and April 2022 were extracted from the US PINC AITM Healthcare Database. Patients who received remdesivir within 2 days of hospitalization were matched 1:1 using propensity score matching to patients who did not receive remdesivir. Additional matching criteria included admission month, age group, and hospital. Cox proportional hazards models were used to examine the effect of remdesivir on risk of 14- and 28-day mortality during VOC periods.
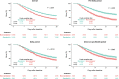
Results: A total of 19 184 remdesivir patients were matched to 11 213 non-remdesivir patients. Overall, 11.1% and 17.7% of remdesivir patients died within 14 and 28 days, respectively, compared with 15.4% and 22.4% of non-remdesivir patients. Remdesivir was associated with a reduction in mortality at 14 (hazard ratio [HR], 0.70; 95% confidence interval, .62-.78) and 28 days (HR, 0.75; 95% CI, .68-.83). The survival benefit remained significant during the pre-Delta, Delta, and Omicron periods.
Conclusions: Prompt initiation of remdesivir in immunocompromised patients hospitalized for COVID-19 is associated with significant survival benefit across all variant waves. These findings provide much-needed evidence relating to the effectiveness of a foundational treatment for hospitalized COVID-19 patients among a high-risk population.
Keywords: COVID-19; comparative effectiveness research; immunocompromised; mortality; remdesivir.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. E. M., E. L., R. G., M. C., and M. B. report employment and being stockholders with Gilead Sciences during the conduct of the study. A. C., S. H. R., and H. J. report funding for study and medical writing provided to their institution (Certara) from Gilead Sciences during the conduct of the study. R. L. G reports serving on scientific advisory boards for AbbVie, Eli Lilly, Gilead Sciences, GSK, Roche, Johnson & Johnson (coronavirus disease 2019 [COVID-19]–related randomized clinical trial, coordinating principal investigator), and Kinevant Sciences (academic steering committee, study investigator; fees to Baylor Scott & White Research Institute); serving as a consultant for Gilead Sciences (honoraria for lectures), Johnson & Johnson, and Kinevant Sciences (through his institution); serving on a speaker bureau for Pfizer unrelated to COVID-19; his institution received a gift-in-kind from Gilead Sciences to facilitate a multicenter clinical trial outside the scope of COVID-19; a de minimis investment in AbCellera; grants or contracts as a study investigator (fees to Baylor Scott & White Research Institute) from Regeneron, Eli Lilly, Gilead, Pfizer, JNJ, and Roivant Sciences (Kinevant Sciences); and receipt of travel support for original scientific presentations from Gilead Sciences. C. C.-M. reports payment or honoraria for lectures/speaker from AstraZeneca and participation on advisory board for Gilead Sciences. A. C. K. reports grants from the National Institutes of Health Adaptive COVID-19 Treatment Trial. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures