Structure Guided Design, Synthesis, and Biological Evaluation of Oxetane-Containing Indole Analogues
- PMID: 37556912
- PMCID: PMC10848874
- DOI: 10.1016/j.bmc.2023.117400
Structure Guided Design, Synthesis, and Biological Evaluation of Oxetane-Containing Indole Analogues
Abstract
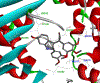
The oxetane functional group offers a variety of potential advantages when incorporated within appropriate therapeutic agents as a ketone surrogate. OXi8006, a 2-aryl-3-aroyl-indole analogue, functions as a small-molecule inhibitor of tubulin polymerization that has a dual mechanism of action as both an antiproliferative agent and a tumor-selective vascular disrupting agent. Replacement of the bridging ketone moiety in OXi8006 with an oxetane functional group has expanded structure activity relationship (SAR) knowledge and provided insights regarding oxetane incorporation within this class of molecules. A new synthetic method using an oxetane-containing tertiary alcohol subjected to Lewis acid catalyzed conditions led to successful Friedel-Crafts alkylation and yielded fourteen new oxetane-containing indole-based molecules. This synthetic approach represents the first method to successfully install an oxetane ring at the 3-position of a 2-aryl-indole system. Several analogues showed potent cytotoxicity (micromolar GI50 values) against human breast cancer cell lines (MCF-7 and MDA-MB-231) and a pancreatic cancer cell line (PANC-1), although they proved to be ineffective as inhibitors of tubulin polymerization. Molecular docking studies comparing colchicine with the OXi8006-oxetane analogue 5m provided a rationale for the differential interaction of these molecules with the colchicine site on the tubulin heterodimer.
Keywords: Friedel-Crafts alkylation; Inhibition of tubulin polymerization; Oxetane.
Copyright © 2023 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

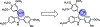




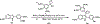
Similar articles
-
Design, Synthesis and Biological Evaluation of 2-Phenyl Indole Analogues of OXi8006 as Colchicine Site Inhibitors of Tubulin Polymerization and Vascular Disrupting Agents.Bioorg Med Chem. 2025 Feb 1;118:117981. doi: 10.1016/j.bmc.2024.117981. Epub 2024 Nov 7. Bioorg Med Chem. 2025. PMID: 39667146
-
Synthesis and biological evaluation of structurally diverse 6-aryl-3-aroyl-indole analogues as inhibitors of tubulin polymerization.Eur J Med Chem. 2024 Jan 5;263:115794. doi: 10.1016/j.ejmech.2023.115794. Epub 2023 Sep 6. Eur J Med Chem. 2024. PMID: 37984295 Free PMC article.
-
Synthesis and Preclinical Evaluation of Indole Triazole Conjugates as Microtubule Targeting Agents that are Effective against MCF-7 Breast Cancer Cell Lines.Anticancer Agents Med Chem. 2021;21(8):1047-1055. doi: 10.2174/1871520620666200925102940. Anticancer Agents Med Chem. 2021. PMID: 32981511
-
Indole molecules as inhibitors of tubulin polymerization: potential new anticancer agents, an update (2013-2015).Future Med Chem. 2016 Jul;8(11):1291-316. doi: 10.4155/fmc-2016-0047. Epub 2016 Jul 20. Future Med Chem. 2016. PMID: 27476704 Review.
-
Indole-Based Tubulin Inhibitors: Binding Modes and SARs Investigations.Molecules. 2022 Feb 28;27(5):1587. doi: 10.3390/molecules27051587. Molecules. 2022. PMID: 35268688 Free PMC article. Review.
References
-
- Hastie SB Interactions of Colchicine with Tubulin. Pharmacol Ther 1991, 51, 377–401. - PubMed
-
- Pettit G; Singh S; Hamel E; Lin C; Alberts D; Garcia-Kendal D. Antitumor Activity of Combretastatin-A4 Phosphate, a Natural Product Tubulin Inhibitor. Experientia 1989, 45, 209–211. - PubMed
-
- Pettit GR; Lippert JW 3rd. Antineoplastic Agents 429. Syntheses of the Combretastatin A-1 and Combretastatin B-1 Prodrugs. Anticancer Drug Des 2000, 15, 203–16. - PubMed
-
- Sriram M; Hall JJ; Grohmann NC; Strecker TE; Wootton T; Franken A; Trawick ML; Pinney KG Design, Synthesis and Biological Evaluation of Dihydronaphthalene and Benzosuberene Analogs of the Combretastatins as Inhibitors of Tubulin Polymerization in Cancer Chemotherapy. Bioorgan Med Chem 2008, 16, 8161–8171. - PubMed
-
- Maguire CJ; Chen Z; Mocharla VP; Sriram M; Strecker TE; Hamel E; Zhou HL; Lopez R; Wang YF; Mason RP; Chaplin DJ; Trawick ML; Pinney KG Synthesis of Dihydronaphthalene Analogues Inspired by Combretastatin A-4 and Their Biological Evaluation as Anticancer Agents. Medchemcomm 2018, 9, 1649–1662. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

