R-loop-dependent promoter-proximal termination ensures genome stability
- PMID: 37557913
- PMCID: PMC10511320
- DOI: 10.1038/s41586-023-06515-5
R-loop-dependent promoter-proximal termination ensures genome stability
Erratum in
-
Author Correction: R-loop-dependent promoter-proximal termination ensures genome stability.Nature. 2025 Feb;638(8049):E1. doi: 10.1038/s41586-025-08606-x. Nature. 2025. PMID: 39833484 Free PMC article. No abstract available.
Abstract
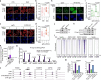
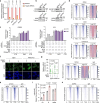
The proper regulation of transcription is essential for maintaining genome integrity and executing other downstream cellular functions1,2. Here we identify a stable association between the genome-stability regulator sensor of single-stranded DNA (SOSS)3 and the transcription regulator Integrator-PP2A (INTAC)4-6. Through SSB1-mediated recognition of single-stranded DNA, SOSS-INTAC stimulates promoter-proximal termination of transcription and attenuates R-loops associated with paused RNA polymerase II to prevent R-loop-induced genome instability. SOSS-INTAC-dependent attenuation of R-loops is enhanced by the ability of SSB1 to form liquid-like condensates. Deletion of NABP2 (encoding SSB1) or introduction of cancer-associated mutations into its intrinsically disordered region leads to a pervasive accumulation of R-loops, highlighting a genome surveillance function of SOSS-INTAC that enables timely termination of transcription at promoters to constrain R-loop accumulation and ensure genome stability.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures













Comment in
-
SOSS-INTAC: a new gatekeeper of genomic integrity at the interface of transcription and R-loops.Mol Cell. 2023 Oct 19;83(20):3593-3595. doi: 10.1016/j.molcel.2023.09.030. Mol Cell. 2023. PMID: 37863028
References
-
- Zheng, H. et al. Identification of Integrator-PP2A complex (INTAC), an RNA polymerase II phosphatase. Science370, eabb5872 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

